Group | Clinical TB Research
Despite progress in the fight against tuberculosis (TB), TB remains a major global health problem. Innovative, high-quality research is needed to combat this poverty-related disease, which primarily affects the most vulnerable.
The mission of our Clinical TB Research Group is to improve the prevention, diagnosis and treatment of TB and its comorbidities especially in high-burden countries. Our portfolio includes clinical trials, intervention studies, translational research and epidemiological studies.
Lines of research
The focus of our research activities is on the following areas:
- Community-based active case finding strategies for TB
- Clinical evaluation of new tools against TB with emphasis on diagnostics and biomarkers
- Host-directed therapies for TB
- Molecular epidemiology
- TB comorbidities

Klaus Reither
PD Dr. med. et phil.
Group Leader
+41612848967
klaus.reither@swisstph.ch
Partners
Key Projects
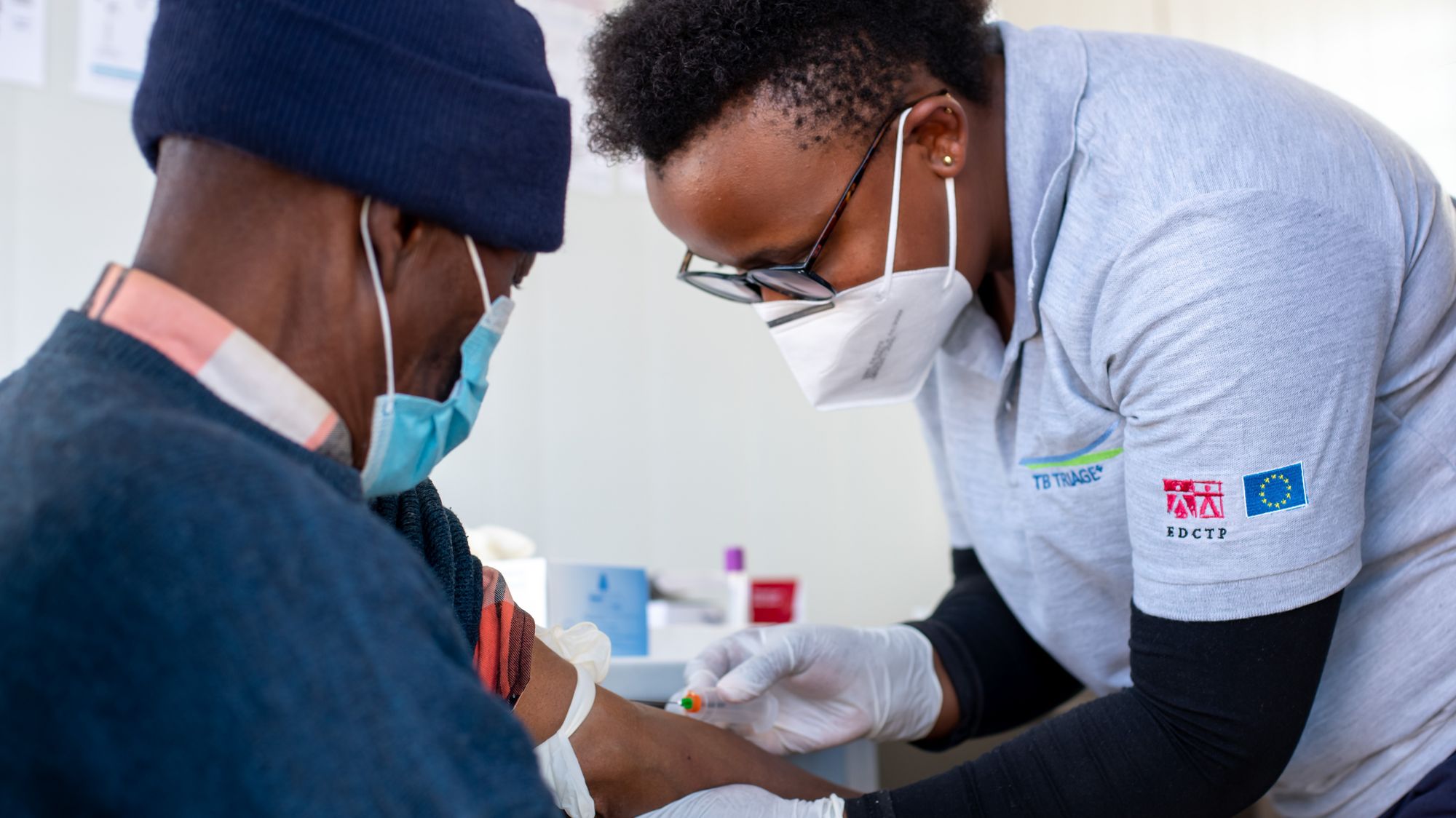
TB Triage+ Community-based tuberculosis triage testing for hard-to-reach populations in Southern Africa
Tuberculosis is a poverty-related disease which particularly affects vulnerable and underserved populations. WHO has developed target product profiles for community-based TB triage testing. CAD4TB, an extensively validated digital chest x-ray analysis platform using deep-learning technology, and a blood test for C-reactive protein, which is a marker of inflammation or infection, are two very promising diagnostic tools which have the potential to meet the targets for TB triage. Read more

MistraL - Mitigation strategies for communities with COVID-19 transmission in Lesotho using artificial intelligence on chest x-rays and novel rapid diagnostic tests
For mitigation strategies to be effective and efficient against COVID-19, they must be context-specific and take local conditions into account. In low- and lower-middle-income countries, limited resources and fragile healthcare systems often dictate what is feasible. In this project, researchers combine artificial intelligence, portable chest X-ray machines and antigen-based diagnostic tests to enable and improve diagnosis of COVID-19 patients in settings with limited resources. Read more

TB-CAPT - Facilitating implementation of TB testing
TB-CAPT (Close the gap, increase Access, Provide adequate Therapy) will provide evidence for impactful implementation of tuberculosis (TB) and TB/HIV co-infection diagnostic strategies including drug-susceptibility testing through a series of trials in Tanzania, Mozambique, and South Africa. TB-CAPT is funded by the European and Developing Countries Clinical Trials Partnership (EDCTP) and will run for 3.5 years, with planned completion in 2023. Read more
Latest Publications
All PublicationsBuziashvili M et al. Analysis of tuberculosis preventive treatment cascade among people with human immunodeficiency virus in Georgia: a mixed-methods study. Open Forum Infect Dis. 2026;13(1):ofaf768. DOI: 10.1093/ofid/ofaf768
Miotto P et al. Clinical evaluation of a commercial culture-free targeted next-generation sequencing test for diagnosis of drug-resistant tuberculosis. Microbiol Spectr. 2026;14(2):e0303525. DOI: 10.1128/spectrum.03035-25
Abongomera C, Keidar O, Paris D.H. Häufig auftretende Krankheiten nach Herkunfts- und Transitländern. In: Exadaktylos A,Keidar O,Srivastava D, eds. Migrations- und Flüchtlingsmedizin, 97-99. Bern: Hogrefe Verlag, 2025
Abongomera C, Labhardt N.D, Paris D.H, Burgener-Gasser A.V. Sexuell übertragbare Infektionen. In: Exadaktylos A,Keidar O,Srivastava D, eds. Migrations- und Flüchtlingsmedizin, 235-244. Bern: Hogrefe Verlag, 2025
Abongomera C, Labhardt N.D, Paris D.H, Burgener-Gasser A.V. Hepatitis B (HBV). In: Exadaktylos A,Keidar O,Srivastava D, eds. Migrations- und Flüchtlingsmedizin, 271-274. Bern: Hogrefe Verlag, 2025
 Teona Avaliani
Teona Avaliani
 Nino Maghradze
Nino Maghradze
 Klaus Reither
Klaus Reither
 Nestani Tukvadze
Nestani Tukvadze