
Melissa Penny is an applied mathematician at Swiss TPH who has worked in public health and infectious disease modelling for over 15 years. She joins us today to discuss the first malaria vaccine, disease modelling and the future of malaria eradication.
Melissa – thanks for joining us! Can you please tell us a bit about yourself?
I am an applied mathematician originally from Australia who has worked in public health and infectious disease modelling for over 15 years, and I’m very interested in using models to inform questions about disease dynamics and public health, and how we might intervene against diseases to reduce burden, for example with vaccines.
The WHO just recommended the widespread use of the malaria vaccine RTS,S: can you talk a bit about this development and the journey to get to this point?
RTS,S has been in development for over 30 years. It was tested in a large phase III clinical trial in Africa, which immunized over 11,000 children in Africa. That achievement itself took a long time to reach with numerous years of research and development and earlier clinical trials. It has involved so many partners, including African research institutes, other research institutes like Swiss TPH, the product development partnership PATH Malaria Vaccine Initiative, the WHO, the Bill & Melinda Gates foundation, as well as many other organizations including the US Army. It was truly a team effort, on an enormous scale.
After the Phase III studies a pilot implementation known as the Malaria Vaccine Implementation Programme has been ongoing and lead by Ghana, Kenya, and Malawi that has reached over 800,000 children since 2019, and was supported by many global partners including funding from Unitaid, Gavi the Vaccine Alliance, PATH, and the WHO. Swiss TPH contributed to both the Phase II and Phase III clinical testing, as well as to understanding immunology dynamics and providing data technology and infrastructure support for the pilot studies. Marcel Tanner, Director Emeritus of Swiss TPH was also involved quite early on and brought a lot of the related projects to the institute, including this particular modelling project.
You contributed to the vaccine coming to fruition through mathematical modelling – what did this look like?
My involvement over the last 13 years in the Disease Modelling unit was during the Phase III clinical studies and later the Malaria Vaccine implementation programme. We were tasked to build models of the likely impact and to estimate the cost-effectiveness of the vaccine. We are very proud that our modelling work, in collaboration with Imperial College London and PATH, could contribute to the recent and very important WHO recommendation.
Modelling was an important part of this recommendation by estimating the effect of the vaccine beyond what was shown in the trial studies, as well as cost-effectiveness estimates. In our models, we took into account demographic and epidemiological data, vaccination coverage rates and people's access to healthcare. We were thrilled when the models found RTS,S has potential to have a high impact in countries with moderate to high malaria transmission. Our work has truly helped to show the impact of the vaccine on saving lives and averting disease.
Throughout this process, was there an ‘Aha moment’ where you realized what you had discovered?
There were a few ‘aha moments’ for our modelling group at Swiss TPH, as well as other modelling groups. Over time, the questions around the vaccines supported a large amount of mathematical model development at Swiss TPH but also other research institutes. In order to make these predictions, we developed not only our open-source individual-based models of malaria, but also a lot of the methodology that goes behind making these economic and impact predictions at global level and for particular geographic settings.
It also included new approaches to analyze Phase III clinical data combined with models of disease dynamics to be able to validate our results and make sure we could make reliable predictions of the likely impact of the vaccine in a wide range of different settings. So there was not one ‘aha moment’ per se, but as a whole, it spawned a lot of new quantitative and model-based research for all other malaria interventions. It also gave rise to a fantastic modelling unit at Swiss TPH, with team members with a range of different biological, medical and quantitative backgrounds collaborating on new projects in infectious disease.
Tell us a bit more about disease modelling; how does it work?
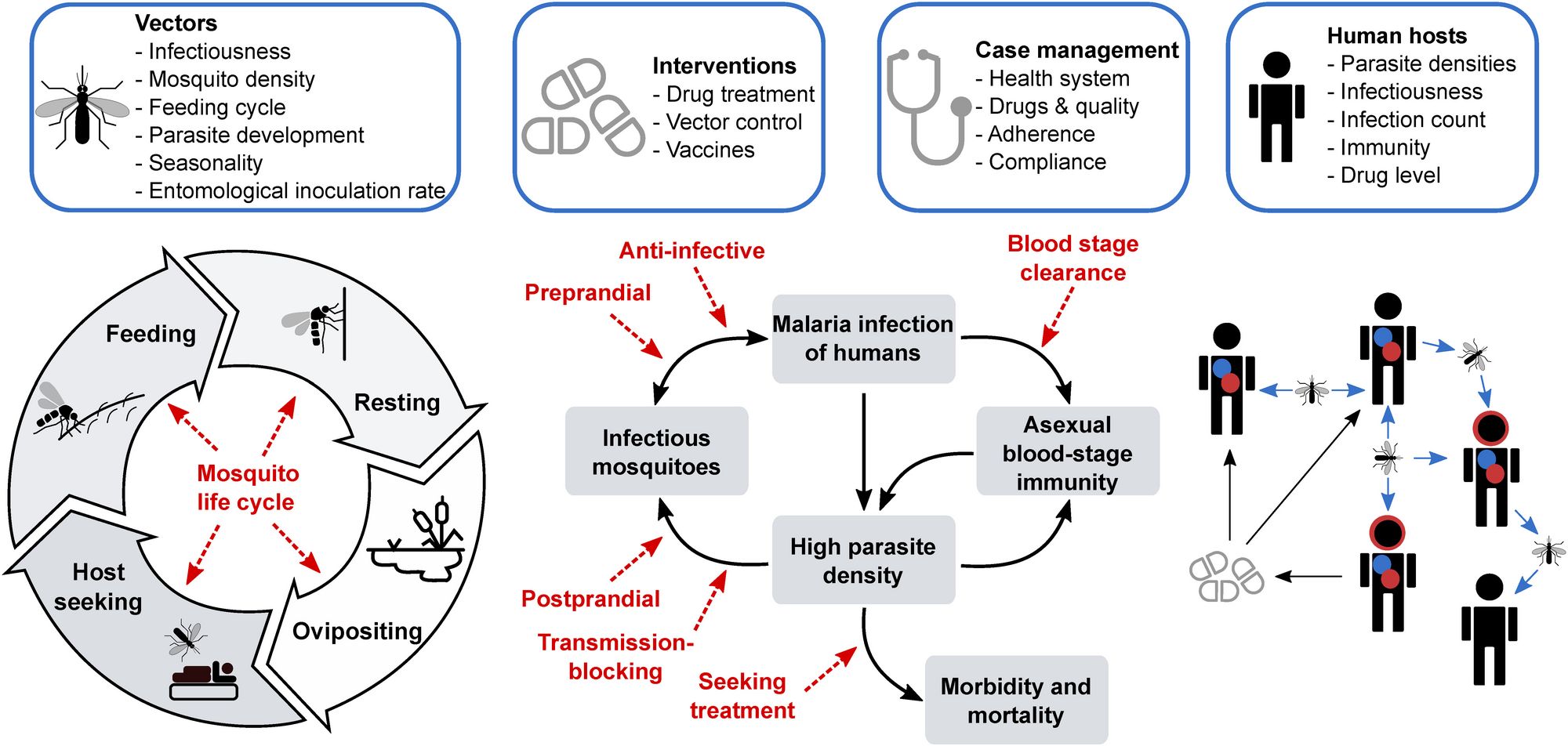
In its simplest form, mathematical modelling takes our observations of say, a disease, and available or known data we have on the disease dynamics, and abstracts these understandings into mathematical equations. Often you use the data to inform or to calibrate some of the parameters in your mathematical equations. You can use these equations to simulate disease dynamics, to both explore the dynamics as well as explore what might happen if you intervene at any part of the disease transmission cycle or at different time points. Basically, you can change the parameters to say, “well what if we break the transmission cycle by giving someone a vaccine?” or, “what happens if we reduce the mosquito density population with bed nets and protect individuals from infected mosquito bites?”
We can use these mathematical representations of the disease to simulate what might happen for a range of different questions. The value of modelling here is not just estimating the impact of the malaria vaccine or a single intervention but we can ask a whole range of questions about malaria disease dynamics, intervention packages and drug resistance for which we do not have data or clinical studies on. These analyses can support thinking and guide discussions on new or existing intervention strategies.

What is the value of modelling beyond modelling for malaria vaccines?
Similar to vaccines, we often ask questions about how we can can reduce the burden of disease. Many of the malaria models that were created to look at malaria vaccines have been used for a wide range of questions since the early vaccine clinical studies and are now used to examine different vector control products, other medical therapeutics such as chemoprevention, to test questions on how effective they might be, how to best deploy them and more. Within the Disease Modelling unit we have a wide number of projects developing new models, exploring new vector control interventions and novel medical interventions, understanding drug resistance dynamics and more. We also have a group at Swiss TPH working on providing analysis of intervention packages for different national malaria control programmes.
Modelling can also be used to answer questions on uncertainties on a diseases, for example with malaria, we can develop and use models to test hypotheses on drug resistance and their impact on the population as well as understanding mechanisms of drug resistance, within a host and between hosts.
What is the future of malaria vaccines?
RTS,S is the first malaria vaccine recommended by WHO for broad use among young children in Africa, but it’s also the first vaccine against a human parasite, which is a major milestone in public health. Next steps will include funding decisions from the global health community for broader rollout, and country decision-making on whether to adopt the vaccine as part of national malaria control strategies.
Other vaccines are being tested which might also go through future recommendations, and there have been strides in the research community to make longer lasting vaccines with a smaller number of doses. And most recently with mRNA vaccination developed in the context of the COVID-19 pandemic, new technologies are now also available for malaria vaccine development in the future.
Aside from modelling, what other projects are you working on?
The beauty of disease modelling and the mathematics behind it is that you can apply it to a wide range of diseases. In the last 18 months, we have also been working on modelling SARS-CoV-2 dynamics and their interaction with vaccines. We worked with the Swiss National Science Task Force in providing potential evidence of the interactions between vaccines and social distancing measures. This data was also shared with the Swiss Federal Office of Public Health (FOPH).
Moving beyond malaria vaccines, we have been working with the Bill and Melinda Gates Foundation to model their next generation medical interventions by providing and using models to give guidance on what completely novel, new medical interventions might look like. For example, monoclonal antibodies, next generation vaccines, and even new interventions to replace chemoprevention.
It’s both an exciting and challenging time for malaria. Progress to reduce malaria burden has stalled in recent years and there is increasing drug and insecticide resistance. At the same time, we have a potential new vaccine available to deploy, and in addition, decades of research and development has resulted in a vast range of novel interventions currently being tested. I’m excited to continue collaborating with all our partners and the fantastic scientists in the Disease Modelling unit at Swiss TPH. To both continue to innovate mathematical and analysis sciences on infectious diseases and malaria, and to contribute to the fight against malaria by using our models to answer pertinent questions on these new interventions and strategies to deploy them.
Photos: M. Kleeb and J. Pelikan, Swiss TPH
Graphic: Disease Modelling Unit, Swiss TPH