Group | Molecular Immunology
The Molecular Immunology group develops new tools to improve disease control of malaria and Mycobacterium ulcerans infection (Buruli ulcer). For both diseases, the group is currently finalising the pre-clinical profiling of candidate vaccines.
For malaria, the vaccine targets the Plasmodium falciparum blood stage protein cysteine-rich protective antigen (PfCyRPA), which forms part of an essential erythrocyte invasion complex. For Buruli ulcer, we are developing a toxoid conjugate vaccine candidate eliciting antibodies that are neutralising the toxic activity of mycolactone, the key virulence factor of Mycobacterium ulcerans.
In the development of point-of-care diagnostics for Buruli ulcer, we are also targeting mycolactone. Our strategy to develop faster and less complex treatments for Buruli ulcer focuses on the repurposing of tuberculosis actives. Screening activities have revealed that the tuberculosis clinical development candidate Telacebek (Q203) has outstandingly high activity against Mycobacterium ulcerans. These findings have provided the rational for first ongoing Buruli ulcer treatment trials with Telacebek.

Gerd Pluschke
Professor, PhD
Group Leader
+41612848235
,
*
gerd.pluschke@swisstph.ch
Malaria
Malaria has remained one of the biggest public health problems and a trend towards reductions in malaria morbidity and mortality has stalled in recent years. Therefore, there is urgent need for an effective malaria vaccine complementing other established anti-malarial control measures. A first and second partially protective malaria vaccine targeting the circumsporozoite protein of Plasmodium falciparum (PfCSP) to prevent P. falciparum malaria in children were approved by the World Health Organization in 2021 and 2023, respectively. However, targeting the sporozoite leaves substantial room for improvement in efficacy and durability, as escape of a single sporozoite into a liver cell can give rise to thousands of blood stage parasites, which do not express PfCSP. Therefore, it is assumed that a vaccine with high efficacy can only be obtained by the addition of a blood stage vaccine component. As the pathology of malaria is caused by the multiplication of the parasites in the bloodstream, major efforts have been made over the past three decades to identify suitable blood stage vaccine candidate antigens. However, none of the handful of blood-stage vaccine candidates clinically tested so far have shown a suitable level of clinical efficacy. This failure is primarily attributed to the high immunogenicity of the tested antigens, which has led to immune escape by the parasite’s development of extensive polymorphisms.
Based on this experience, we have embarked on the identification of gaps in immune recognition, i.e. the identification of essential blood stage antigens showing limited immunogenicity in the natural context. By screening uncharacterized proteins expressed in late blood stage for their ability to generate parasite-inhibitory antibodies, we have identified the P. falciparum cysteine-rich protective antigen (PfCyRPA) (Dreyer et al, 2012). PfCyRPA shows only very limited immunogenicity in the natural context. As a consequence, the PfCyRPA sequence is highly conserved. However, if the protein is delivered in an appropriate formulation, it is capable of inducing strain-transcending parasite growth inhibitory antibodies.
Selected Projects
Elucidating the Function of PfCyRPA
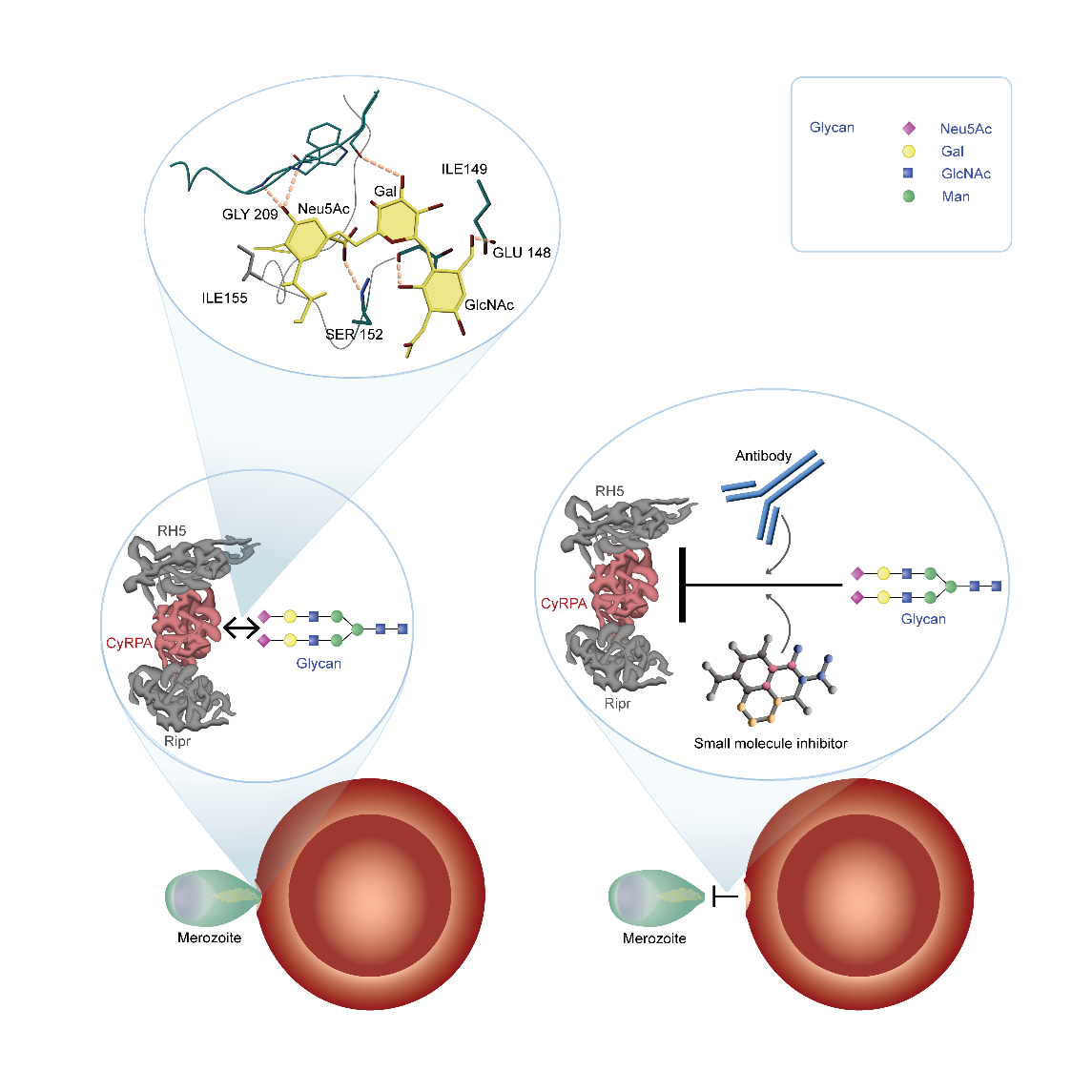
PfCyRPA forms together with other proteins an essential erythrocyte invasion complex. Assembly of the invasion complex at the apical end of the merozoites is leading to the insertion of the complex into the erythrocyte membrane, which is initiating the following steps of invasion. Underpinned by results of a crystal structure analysis of PfCyRPA and its complex with a parasite invasion inhibitory antibody (Favuzza at al. 2017), we found that PfCyRPA is a lectin targeting glycans terminating with α2-6-linked N-acetylneuraminic acid (Day et al., 2024). Lectin sites were validated by mutagenesis studies and inducible transgenic parasite lines expressing endogenous PfCyRPA with reduced glycan binding activity showed that the lectin activity plays an important role in parasite invasion. Accordingly, blocking of PfCyRPA-glycan ligand binding is an effective strategy for malaria blood stage vaccination.
Development of PfCyRPA-based Malaria Vaccine Candidate
In a first phase, we have developed two vaccine formulations based on recombinantly expressed PfCyRPA protein (Tamborrini et al. 2020, 2023), which demonstrated that PfCyRPA can elicit high titers of parasite-inhibitory antibodies if it is delivered in a suitable formulation. Motivated by the success and cost-effectiveness of the COVID mRNA vaccines, we have more recently embarked on the development of a PfCyRPA-based mRNA vaccine, with the hope of achieving higher antibody titers than with the protein-based vaccine approach. We have selected an optimized codon-adapted PfCyRPA mRNA sequence and both in mice and rabbits, we have observed excellent immunogenicity with a well characterized LNP formulation of this sequence. One immunization with the optimized mRNA vaccine candidate elicited anti-PfCyRPA antibody titers comparable to titers only seen after two or three immunizations with PfCyRPA recombinant protein-based vaccine candidates. After a second immunization with the mRNA we saw titers which we never reached with any of the protein-based immunizations. The mRNA vaccine candidate thus clearly outperforms both protein-based vaccine formulations. We use standardized parasite growth inhibitory activity (GIA) in vitro assays to assess the activity of the elicited rabbit anti PfCyRPA antibodies. While purified pre-immunization IgG showed no significant growth inhibitory activity, IgG from all immunized rabbits showed substantial activity with IC50 values corresponding to a 50-fold dilution of the sera. Based on the very promising GIA data, we are developing plans for a first clinical concept validation in a phase 1a/2a controlled P. falciparum blood stage human challenge trial.
Related Publications
Day C.J et al. The essential malaria protein PfCyRPA targets glycans to invade erythrocytes. Cell Rep. 2024;43(4):114012. DOI: 10.1016/j.celrep.2024.114012
Tamborrini M, Schäfer A, Hauser J, Zou L, Paris D.H, Pluschke G. The malaria blood stage antigen PfCyRPA formulated with the TLR-4 agonist adjuvant GLA-SE elicits parasite growth inhibitory antibodies in experimental animals. Malar J. 2023;22:210. DOI: 10.1186/s12936-023-04638-8
Favuzza P et al. Structure of the malaria vaccine candidate antigen CyRPA and its complex with a parasite invasion inhibitory antibody. eLife. 2017;6:e20383. DOI: 10.7554/eLife.20383
Dreyer A.M et al. Passive immunoprotection of Plasmodium falciparum-infected mice designates the CyRPA as candidate malaria vaccine antigen. J Immunol. 2012;188(12):6225-6237. DOI: 10.4049/jimmunol.1103177
Buruli Ulcer
After tuberculosis and leprosy, Buruli ulcer, caused by M. ulcerans, is the third most common mycobacterial disease, and Western Africa is the world region most affected by this chronic necrotising disease of the skin and the subcutaneous tissue. M. ulcerans is unique among mycobacterial pathogens in that it is mainly extracellular and produces a plasmid-encoded toxin with a polyketide-derived macrolide structure, named mycolactone.
Symptoms and Treatment
Mycolactone is believed to play a central role in determining the extracellular localization of the bacteria and modulation of immunological responses to M. ulcerans. Clinical lesions usually start as painless nodules and if left untreated lead to massive destruction of skin and sometimes bone. While surgery has traditionally been the only recommended treatment for BU, in 2004 WHO published provisional guidelines recommending treatment with a combination of rifampicin and streptomycin for 8 weeks.
Our Research
We developed a broad research portfolio comprising clinical, field and laboratory studies. The goals of our research are to improve understanding of the pathogenesis, immunology and transmission of Buruli ulcer, develop methods for early diagnosis, and investigate prospects for improving therapy and vaccine development.
Related Publications
Pluschke G, Warryn L. How our molecular understanding of the pathogenesis of Mycobacterium ulcerans infection can improve diagnosis of Buruli ulcer. Expert Rev Mol Diagn. 2023(1-2):1-4. DOI: 10.1080/14737159.2023.2294333
Warryn L, Pluschke G. Repurposing of tuberculosis drug candidates for the treatment of Mycobacterium ulcerans disease. Chimia (Aarau). 2023;77(9):577-581. DOI: 10.2533/chimia.2023.577
Warryn L, Dangy J.P, Gersbach P, Gehringer M, Altmann K.H, Pluschke G. An antigen capture assay for the detection of mycolactone, the polyketide toxin of Mycobacterium ulcerans. J Immunol. 2021;206(11):2753-2762. DOI: 10.4049/jimmunol.2001232
Thomas S.S, Kalia N.P, Ruf M.T, Pluschke G, Pethe K. Toward a single-dose cure for Buruli ulcer. Antimicrob Agents Chemother. 2020;64(9):e00727-20. DOI: 10.1128/AAC.00727-20
Warryn L et al. Development of an ELISA for the quantification of mycolactone, the cytotoxic macrolide toxin of Mycobacterium ulcerans. PLoS Negl Trop Dis. 2020;14(6):e0008357. DOI: 10.1371/journal.pntd.0008357
Scherr N et al. Targeting the Mycobacterium ulcerans cytochrome bc1:aa3 for the treatment of Buruli ulcer. Nat Commun. 2018;9:5370. DOI: 10.1038/s41467-018-07804-8
Links and Collaborations
Meningitis
Meningococcal Meningitis: Clonal Waves of Colonisation and Disease in the Meningitis Belt of Sub-Saharan Africa
Bacterial meningitis is a medical emergency and remains one of the major health problems in Sub-Saharan Africa. The three most important agents are Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae. All three pathogens are common colonizers of the human nasopharynx, invasive disease is usually a rare event.
The bacterial meningitis project involves longitudinal studies of the molecular epidemiology of comparatively carriage and disease of N. meningitidis and S. pneumoniae. The studies are being carried out in Northern Ghana and Burkina Faso and aim to enhance the understanding of the dynamics of meningitis epidemics in the African Meningitis Belt. The results provide important background information for the evaluation and introduction of new conjugate vaccines against meningococcal and pneumococcal infections in Africa.
Related Publications
GBD 2019 Meningitis Antimicrobial Resistance Collaborators. Global, regional, and national burden of meningitis and its aetiologies, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2023;22(8):685-711. DOI: 10.1016/s1474-4422(23)00195-3
Jen F.E, Abrahams J.L, Schulz B.L, Lamelas Cabello A, Pluschke G, Jennings M.P. High-frequency changes in pilin glycosylation patterns during Neisseria meningitidis serogroup a meningitis outbreaks in the African meningitis belt. ACS Infect Dis. 2023;9:1451–1457. DOI: 10.1021/acsinfecdis.3c00149
Lamelas A et al. Emergence and genomic diversification of a virulent serogroup W:ST-2881(CC175) Neisseria meningitidis clone in the African meningitis belt. Microb Genom. 2017;3(8):e000120. DOI: 10.1099/mgen.0.000120
Lamelas A et al. Emergence of a new epidemic Neisseria meningitidis serogroup A clone in the African meningitis belt: high-resolution picture of genomic changes that mediate immune evasion. mBio. 2014;5(5):e01974-14. DOI: 10.1128/mBio.01974-14


 Julia Hauser
Julia Hauser
 Gerd Pluschke
Gerd Pluschke
 Louisa Warryn
Louisa Warryn