Clinical Trials Completed for a New Drug to Treat Parasitic Worm Infections in Infants
16.11.2021
The Pediatric Praziquantel Consortium completed its pivotal clinical Phase III trial of arpraziquantel – a potential new treatment option for the estimated 50 million preschool-aged children with schistosomiasis. As a member of the Consortium, Swiss TPH led the implementation of the clinical trials in Côte d’Ivoire and Kenya.
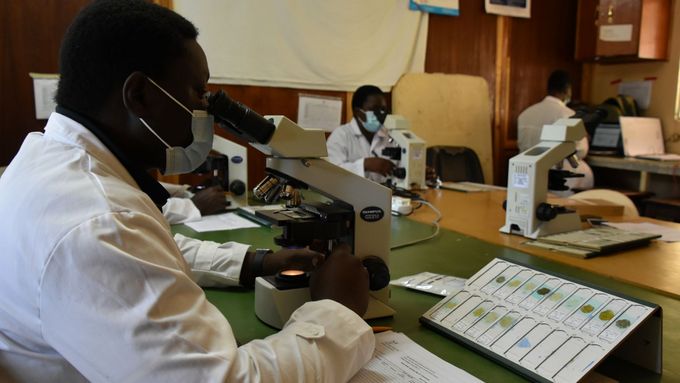
Technician analysing stool samples from the clinical trial for parasite eggs, Homa Bay, Kenya. (Photo: Eric Huber, Swiss TPH)
Today, the Pediatric Praziquantel Consortium, a public-private partnership dedicated to the development of arpraziquantel, a new treatment option for schistosomiasis in preschool-aged children, announced the completion of its pivotal Phase III trial in Côte d'Ivoire and Kenya.
The results of the trial, co-funded by the Global Health Innovative Technology (GHIT) Fund and the European & Developing Countries Clinical Trials Partnership (EDCTP), confirm a favorable efficacy and safety profile for arpraziquantel in children 3 months to 6 years of age, affected by this neglected tropical disease (NTD). This allows the program to progress towards regulatory file submission to the European Medicines Agency (EMA).
Addressing unmet needs for preschool-aged children
Schistosomiasis is one of the most damaging NTDs caused by parasitic worms. It affects the lives of around 240 million people. It is highly prevalent in sub-Saharan Africa where it causes anaemia, stunted growth, reduced ability to learn and chronic inflammation of the organs, which can be fatal.
The current standard treatment for schistosomiasis is the drug praziquantel. It is suitable for school-aged children and adults. To date, preschool-aged children are not included in treatment programmes, primarily due to the lack of a child-friendly formulation of the drug. Still, they are highly affected. “The new medication will be instrumental in the fight against schistosomiasis in preschool-aged children, previously left untreated under public health programmes,” said Jennifer Keiser, Head of the Helminth Drug Development unit at Swiss TPH and member of the board of the Pediatric Praziquantel Consortium.
Derived from praziquantel, the new pediatric medication arpraziquantel is an orally dispersible tablet. It is small, has appropriate taste properties, can be taken with or without water, and withstands the hot and humid conditions presented by a tropical climate.
Clinical trials in Côte d’Ivoire and Kenya
Swiss TPH is a founding member of the Pediatric Praziquantel Consortium and has been leading the implementation of clinical trials in endemic countries in Africa from phase I to III since 2015. The phase III trial was implemented in Côte d’Ivoire and Kenya. Children aged 3 months to 6 years infected with intestinal or urinary schistosomiasis were enrolled in different age groups and treated with a single dose of arpraziquantel. Stool and urine samples were analysed for parasite eggs. High efficacy was observed with cure rates close to or above 90%.
Arpraziquantel treatment demonstrated favorable safety, tolerability and improved palatability among preschool-aged children. No new potential risks or safety concerns were identified. “It is a great achievement that despite disruptions due to COVID-19, we were able to continue and complete the clinical trials so that the whole package of data collected can now be submitted for the registration of this new tablet,” said Eric Huber, Project Leader of the clinical trials at Swiss TPH.
Preparing for access and delivery of the new medication
With the full clinical development phase successfully completed, the programme has entered the regulatory filing stage, while preparing for the potential delivery of arpraziquantel through the consortium’s dedicated access programme, ADOPT. It aims to support the introduction of the drug into routine practice in community settings and provide a practical toolkit to guide implementation in endemic countries along with high-level advocacy and demand forecast. “Thanks to joint efforts of several partners from the public and the private sector, a child-friendly formulation will finally be available. We are now redoubling our efforts to allow the vulnerable population of preschool-aged children to be included in treatment programmes,” said Peter Steinmann, public health specialist and coordinator of the ADOPT programme at Swiss TPH.
Contact

Jennifer Keiser
Associate Professor, PhD
Head of Unit
+41612848218
jennifer.keiser@swisstph.ch

Eric Huber
MSc
Project Leader
+41612848972
eric.huber@swisstph.ch
Stay connected
Subscribe to our newsletter and get all the latest research news, project updates, course and event listings from Swiss TPH.
