Malaria
P. falciparum Malaria: Developing a Synthetic Subunit Candidate Vaccine
Malaria is one of the most serious infectious diseases of humans, infecting 5–10% of the world’s population, with 300–600 million clinical cases and more than 2 million deaths annually. Moreover, malaria is a major social and economic burden in endemic areas. In recent years, malaria has spread at an alarming rate owing to the increasing resistance of the parasite to drugs, and the resistance of mosquitoes to insecticides. Therefore, new approaches to combat malaria are urgently needed, and a vaccine is predicted to have the greatest impact in addition to being the most cost-effective control measure.
Vaccine Development
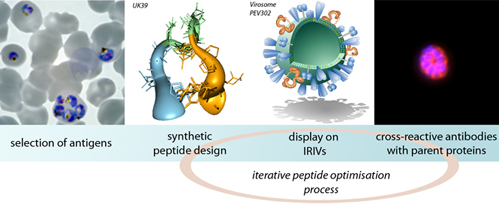
One approach is to design a subunit vaccine that incorporates several malaria protein antigens for which there is evidence of protective immunity from epidemiological data or experimental animal challenge models. Development of such subunit vaccines is critically dependent on the availability of an antigen delivery system to drive suitable protein antigen-specific immune responses in humans. Vaccine formulations have to be highly effective, human-compatible and safe. Production of synthetic or recombinant proteins that stably mimic the native structure of the corresponding malaria antigens to induce effective humoral immune responses is a further major challenge.
Our research
We are addressing both problems by developing synthetic peptide structures that induce cross-reactive antibodies against the parent malaria proteins and by coupling them to the surface of immunopotentiating reconstituted influenza virosomes (IRIVs). In addition we are evaluating the use of so fare uncharacterized predicted proteins of Plasmodium falciparum as potential new candidate vaccine antigens.
Selected Publications
Abubakar A.T et al. Prospects for dog rabies elimination in Nigeria by 2030. Zoonoses Public Health. 2024;71(in press). DOI: 10.1111/zph.13084
Agunbiade O.M et al. Social context of intimate partner violence and system response during COVID-19 in Africa: a scoping review. Int J Popul Stud. 2024;10(1):367. DOI: 10.36922/ijps.367
Ajayi O et al. Discovery of an orally active nitrothiophene-based antitrypanosomal agent. Eur J Med Chem. 2024;263:115954. DOI: 10.1016/j.ejmech.2023.115954
Alkhaldi M, Meghari H, AlBada M. Rethinking the World Health Organization's leadership of global health governance and the global health surveillance systems. Glob Health Promot. 2024(in press). DOI: 10.1177/17579759231220529
Arnaiz P et al. Acceptability and perceived feasibility of the KaziKidz health promotion intervention among educators and caregivers in schools from South Africa: a qualitative descriptive study. BMC Public Health. 2024;24:934. DOI: 10.1186/s12889-024-18456-3
Arnskötter W, Martin S, Walitza S, Hediger K. Effects of including a dog on treatment motivation and the therapeutic alliance in child and adolescent psychotherapy: study protocol for a randomized controlled trial. Trials. 2024;25(1):26. DOI: 10.1186/s13063-023-07854-4
Arsenault C et al. Health system quality and COVID-19 vaccination: a cross-sectional analysis in 14 countries. Lancet Glob Health. 2024;12(1):e156-e165. DOI: 10.1016/s2214-109x(23)00490-4
Aryal A, Clarke-Deelder E, Phommalangsy S, Kounnavong S, Fink G. Health system utilization and perceived quality among adults in Lao PDR: evidence from a nationally representative phone survey. BMC Public Health. 2024;24:565. DOI: 10.1186/s12889-024-18039-2
Avezum Á et al. An intersectoral approach to hypertension care: solutions for improving blood pressure control in São Paulo, Brazil. Am J Hypertens. 2024(in press). DOI: 10.1093/ajh/hpae005
Baert L et al. Induced pluripotent stem cell-derived human macrophages as an infection model for Leishmania donovani. PLoS Negl Trop Dis. 2024;18(1):e001155. DOI: 10.1371/journal.pntd.0011559