The phase II/III CALINA study evaluated the safety and efficacy of artemether-lumefantrine in infants weighing less than 5 kg. In the DRC, over 14,000 children were screened for potential enrolment, making it one of the most active sites in the study.
Conducting paediatric clinical trials in low-resource settings is complex – from infrastructure and logistics to staff training and community engagement. The success of the DRC site reflects strong collaboration on the ground and with partners and draws on many years of experience in clinical research in similar contexts.
In this interview, team members from both the DRC and Allschwil share their perspectives. They reflect on what it takes to prepare and run a clinical trial in such a setting, how they experienced the study on a personal and professional level, and why the recent regulatory approval is such a meaningful development.
Setting the scene
Why was it important to include the DRC in this trial?
Yves Lula, Clinical Research Manager: Malaria remains a major public health concern in the DRC, with children weighing less than 5 kg being among those most affected. The DRC experiences all three types of malaria, which exhibit relatively high genetic diversity.
How does this site reflect the real-world context in which the drug will be used?
Malaria disproportionately affects vulnerable populations living in rural areas with limited resources and difficult access to health centres. The study site in Kisantu largely encompasses this demographic.
What does this approval mean for infants in malaria-endemic countries?
This approval marks a significant and long-awaited change in the management of newborns. The current treatment with quinine drops is inadequate: it requires seven days, the solution is unstable once opened, and costs rise when combined with clindamycin.

“This approval marks a significant and long-awaited change in the care of newborns.”
Yves Lula
Clinical Research Manager, Clinical Operations, Swiss TPH
Building a trial site in a challenging context
What were the biggest logistical or infrastructure challenges at the DRC site?
Stefan Schneitter, Project Leader: Bringing the site’s infrastructure up to the required standard was challenging. Prior to the trial, infrastructure investments were necessary. We had to renovate and set up the laboratory, as well as install equipment ranging from air conditioning to biochemistry and haematology equipment. In addition, the patients’ ward, pharmacy and office space required renovation.

How do you build trust and train local teams for a study like this?
Morgane Nusbaumer, Lead Clinical Research Associate: We are fortunate to already have a long history of working with DRC, so we are very familiar with the local context. Trust is indeed the most important value when setting up and conducting a study remotely, especially as we started during the pandemic and could not travel. There is no mystery to building trust – it takes time. Fortunately, we had a solid, dedicated and skilled local team in place that we knew well and could rely on; they helped us to implement the study despite difficult circumstances.

“Fortunately, we had a solid, dedicated and skilled local team in place that we knew well and could rely on.”
Morgane Nusbaumer
Lead Clinical Research Associate, Clinical Operations, Swiss TPH
Patient Pathway: From Screening to Care
What does a typical patient journey look like during the trial – from screening to enrolment to follow-up?
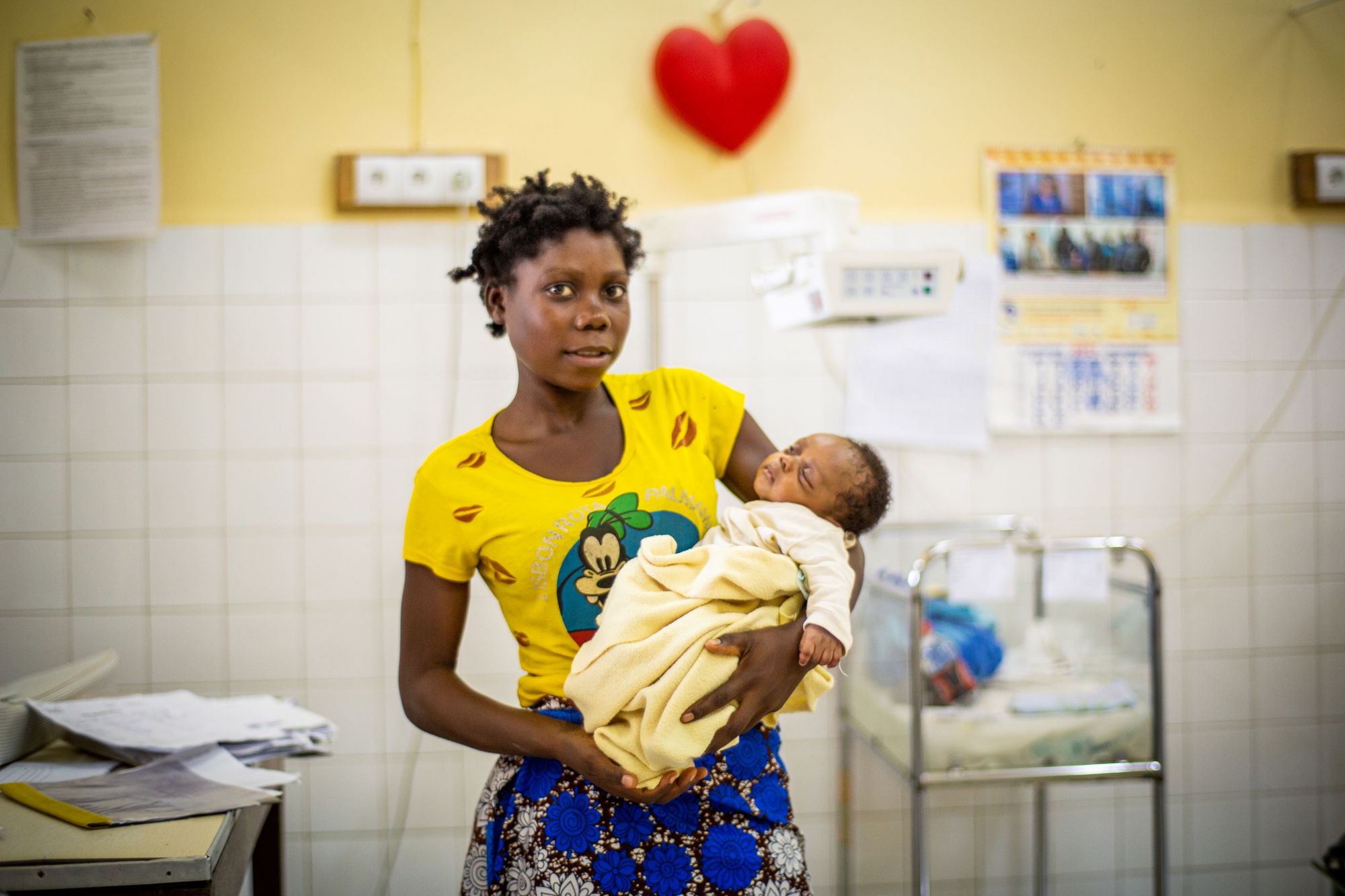
Freia Nicolás, Clinical Research Associate: Most of the participants were referred from health centres, because, in the DRC, people with simple malaria usually go to health centres rather than hospitals. After providing informed consent and checking the inclusion/exclusion criteria, participants stayed in hospital with their mothers for four days, corresponding to the three days of treatment and the first follow-up visit. Then they returned for follow-up visits on days 5, 8, 15, 39 and 43, and again at one year of age. During these visits, malaria blood smears were normally performed alongside other physical and blood tests. A neurodevelopment assessment was conducted during the final visit at one year of age.
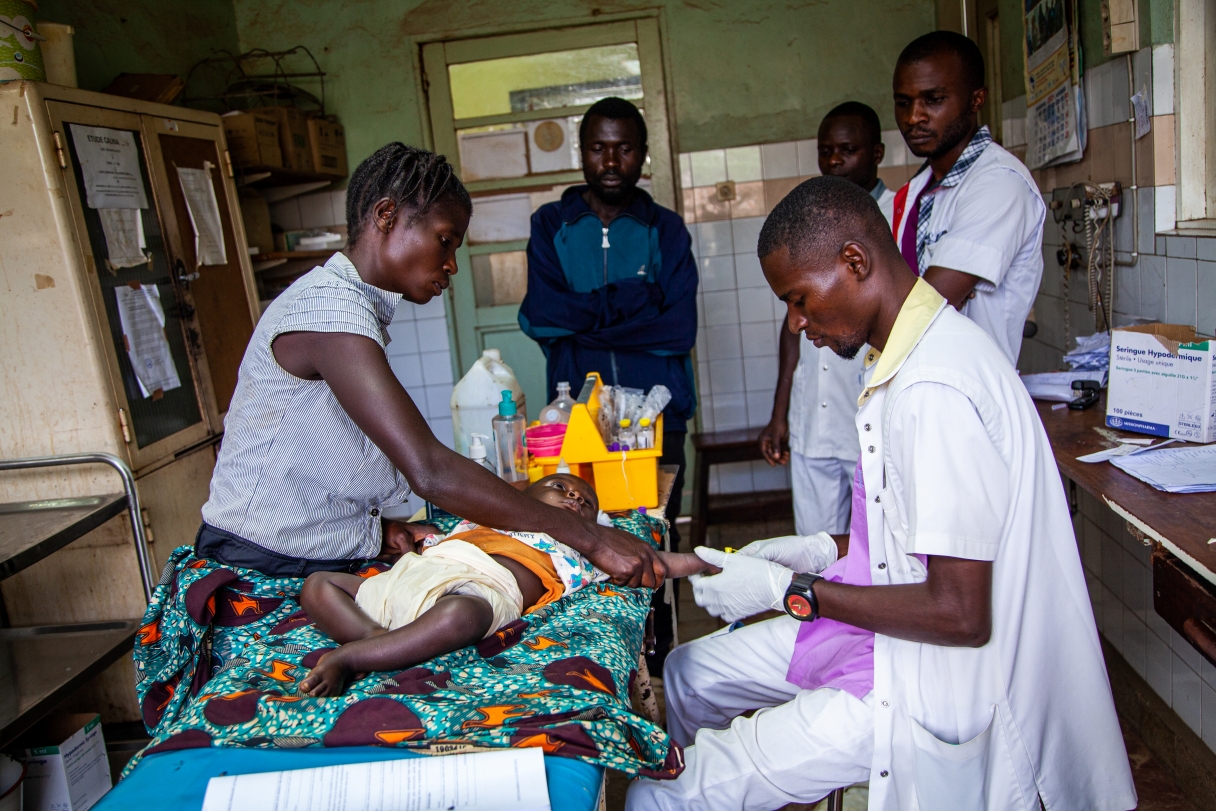

“Building parents’ trust was key to enrolling these young children, as was addressing their concerns.”
Freia Nicolás
Clinical Research Associate, Clinical Operations, Swiss TPH
What precautions or safeguards are in place when working with such a vulnerable population?
The strict regulations for blood draws at this age prompted us to carefully balance meeting the sponsor’s requirements with ensuring minimal blood sampling to guarantee the safety and well-being of the participants. Building parents’ trust was key to enrolling these young children, as was addressing their concerns about follow-up visits and blood draws respectfully. As the participants were so young, a parent stayed with them in the hospital for four days, so ensuring the parents’ comfort – such as providing meals – was an important part of supporting the families throughout their stay.
Science & Data: Swiss TPH’s Contribution
What was Swiss TPH’s role in the molecular analyses for CALINA?
Christian Nsanzabana, Scientific Group Leader: As part of the molecular analyses work in CALINA, Swiss TPH examined the parasite’s DNA to gain a better understanding of infections and treatment effects. We performed PCR genotyping and resistance marker analysis on clinical samples. These steps help to identify whether infections are new or recurring, and whether the parasites carry changes linked to drug resistance.
How does the lab work support and strengthen the trial results?
By confirming which cases of malaria were new infections and checking for resistance markers, the molecular work provides scientific certainty to the trial’s findings. This ensures that the results regarding the safety and effectiveness of the treatment are accurate and reliable.

“We process, analyse and store all samples at Swiss TPH to the same strict standards, regardless of their origin.”
Christian Nsanzabana
Scientific Group Leader, Genotyping, Swiss TPH
How did you ensure data quality and protocol compliance across all partner sites?
We maintained high quality standards by always treating samples from all sites in the same way – using the same protocol, the same treatment steps, the same data analysis and reporting. Regardless of their origin, we process, analyse and store all samples at Swiss TPH to the same strict standards. Consequently, the results are solid and comparable across the entire study.

Collaboration Across Continents
How did the teams in Allschwil and DRC work together during the trial?
Morgane: We held weekly meetings with our local lead clinical research associate to follow up on the study. At the same time, communication needed to be flexible, so we also set up a WhatsApp group with the local team to handle urgent questions and requests. Of course, no sensitive information or data was shared, but this channel enabled faster, more practical coordination alongside formal communication.
What was the collaboration like with local health authorities?
Freia: Throughout the project, close collaboration with the local health authorities was essential. Export/import licenses were required for shipping samples, investigational medicinal products (IMP) and other materials, and the sponsor’s requirements also had to be met. Out local support was crucial to the success of this collaboration.
How does Swiss TPH’s global network help make trials like CALINA possible?
Stefan: To conduct such a complex trial under these conditions, a strong local team was essential. Our team was in constant contact with the study site and with local third-party providers for laboratory support and equipment supply.
During the planning phase, the Swiss TPH network played a key role in identifying a suitable country and site. Thanks to a thorough assessment, we were able to find a site with the target patient group (babies weighing less than 5kg), where more than 80% of all trial participants were ultimately recruited.

Personal Reflections from the Team
What makes you proud of this project?
Freia: Through this project, I feel that my work made a tangible difference: strengthening hospital staff capacity and helping develop a medicine tested in the very region and among the vulnerable population where it is most needed. Despite all the challenges involved, the drug has now obtained market authorization.
Looking Ahead
What lessons from CALINA can we take forward to future trials?
Stefan: You have to go where the patients are. This is especially true for trials requiring a very specific patient population. Even if this means investing in infrastructure and human resources prior to the trial. Continuous capacity strengthening of on-site staff remains essential. And since the site often recruited patients in the evening, the Swiss TPH teams in DRC and Switzerland also needed to be available to answer questions outside office hours.

“You have to go where the patients are. This is especially true for trials requiring a very specific patient population.”
Stefan Schneitter
Project Leader, Clinical Operations, Swiss TPH