Helminth Immunology
Hookworm (Necator americanus or Ancylostoma duodenale) infections represent a major neglected tropical disease, affecting 700 million people worldwide and causing morbidity due to their impact on nutrient uptake and their need to feed on host blood resulting in severe anemia. Hookworm mainly infects people living in impoverished conditions without access to adequate sanitation and is characterized clinically by anemia, malnutrition in pregnant women, and an impairment of the growth and cognitive development of children.
The Helminth Immunology group conducts basic and preclinical research aiming to enhance our understanding of host pathogen interactions with the long-term goal to identify new strategies of intervention against hookworms. We use a multidisciplinary approach combining traditional parasitology, molecular parasitology and immunology to uncover the cellular and molecular events involved in parasite evasion of host immune attack, as well as weaknesses in parasite development that can be targeted with drugs.
We study helminth parasites, because they are fascinating and complex organisms that have co-evolved with us for so long that they have marked deeply our immune system.
We believe our work can :
- Alleviate infection burden in hookworm infected people, as well as be some related veterinary disease (such as Haemonchus infections in sheep and cattle)
- Help understand and control inflammatory diseases that arise in absence of helminth infection (such as allergies or autoimmune diseases)
We have currently three axis of research:
- Deciphering host parasite/interactions: from microbiome to pathology
- Developing novel anthelmintics and vaccine
- Understanding parasites development through molecular approaches
Selected projects
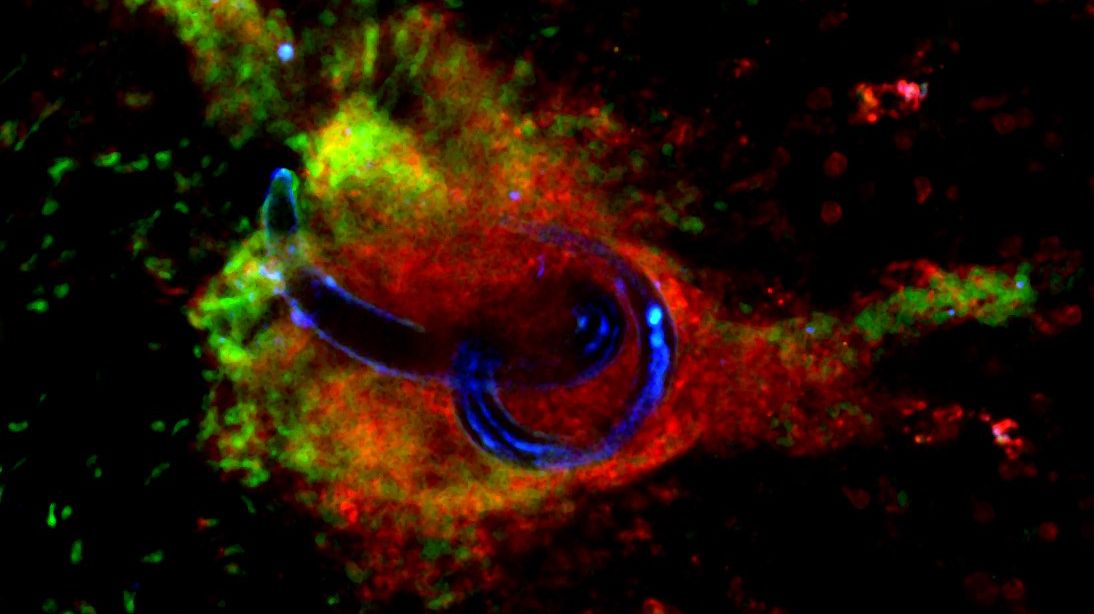
Strategies for Hookworm Eradication (SHE): Harnessing the parasite developmental keys for designing targeted clinical approaches
The overarching goal of this research project is to identify new intervention strategies against soil-transmitted helminths, and in particular against hookworms. More specifically, the research programme will focus on two complementary approaches: i) the identification of compounds that can be used as drugs, which target biological pathways of importance in the development, establishment and survival of soil-transmitted helminths, and ii) the identification of new vaccine targets that could be used to block the establishment of the parasite. Read more
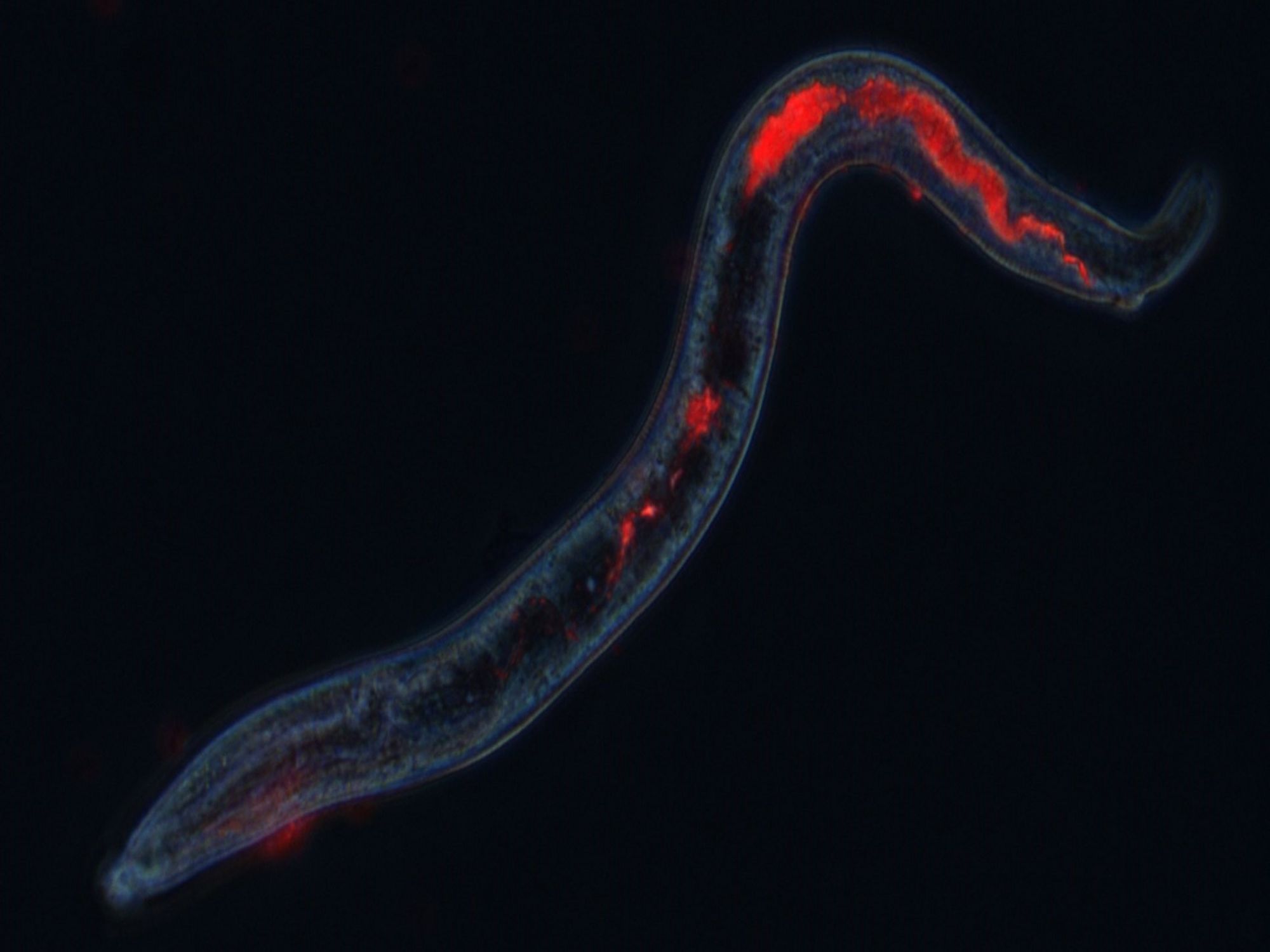
Targeted drug discovery against blood-feeding parasite nematodes of animals
This project led by Monash University aims to identify more sustainable control strategies of nematode parasites of livestock, which cost more than 400 million yearly to the Australian wool and meat industry. The project expects to identify novel nematicides and generate knowledge of the parasite biology using a combination of high-throughput drug discovery screens with cutting-edge OMICs approaches to target a key molecular pathway of importance to the survival of nematodes, namely their blood-feeding behaviour. Tiffany Bouchery is partner investigator in this project. Read more
Bouchery T., Volpe B., Doolan R., Coakley G., Moyat M., Esser-von Bieren J., Wickramasinghe L.C., Hibbs M., Sotillo J., Camberis M., Le Gros G., Khan N., Williams D., Harris N./ β-glucan receptors on IL-4 activated macrophages are required for hookworm larvae recognition and trapping. Immunology and Cell Biology. https://doi.org/10.1111/imcb.12536
Bouchery T., Moyat M, Sotillo J., Silverstein S., Volpe B., Coakley G., Tsourouktsoglou TD.,Becker L., Shah K.,, Kulagin M., Guiet R., Camberis M., Schmidt A., Seitz A., Giacomin P.,LeGros G., Papayannopoulos V., Loukas A., Harris N. / Hookworms evade host immunity by secreting a deoxyribonuclease to degrade neutrophil extracellular traps. Cell Host and Microbes. 2020. https://doi.org/10.1016/j.chom.2020.01.011
Bouchery, T ; Filbey, K ; Shepherd, A .; Chandler, J. ; Patel, Deepa ; Schmidt, Alfonso ; Camberis, Mali ; Peignier, Adeline ; Smith, Adam A.T. ; Johnston, K. ; Painter, G. ; Pearson, M. ; Giacomin, P.; Loukas, A. ; Bottazzi, ME.; Hotez, P.; LeGros, G. / A novel blood-feeding detoxification pathway in Nippostrongylus brasiliensis L3 reveals a potential checkpoint for arresting hookworm development. In: PLoS Pathogens. 2018; Vol. 14, No. 3. https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1006931
Cardoso, V.; Chesné, J.; Ribeiro, H.; Garcia-Cassani, B.; Carvalho, T.; Bouchery, T.; Shah, K.; BarbosaMorais, N.; Harris, N.; Veiga-Fernandes, H. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. In: Nature. 2017; Vol. 549, No. 7671. pp. 277-281. https://www.nature.com/articles/nature23469
Bouchery, T.; Volpe, B.; Shah, K.; Lebon, L.; Filbey, K.; LeGros, G.; Harris, N. / The Study of Host Immune Responses Elicited by the Model Murine Hookworms Nippostrongylus brasiliensis and Heligmosomoides polygyrus. In: Current Protocols in Mouse Biology. 2017; Vol. 7, No. 4. pp. 236-286. https://pubmed.ncbi.nlm.nih.gov/29261231/
Bouchery, T.; Kyle, R. ; Camberis, M. ; Shepherd, A. ; Filbey, K. ; Smith, A. ; Harvie, M. ; Painter, G. ; Johnston, K. ; Ferguson, P. ; Jain, R. ; Roediger, B. ; Delahunt, B.; Weninger, W. ; Forbes-Blom, E. ; Le Gros, G. ILC2s and T cells cooperate to ensure maintenance of M2 macrophages for lung immunity against hookworms. In: Nature Communications 2015; Vol. 6. https://www.nature.com/articles/ncomms7970

 Tiffany Bouchery
Tiffany Bouchery
 Dovran Ovezgeldiyev
Dovran Ovezgeldiyev