Unit | Helminth Drug Development
The Helminth Drug Development Unit focuses on the discovery, development and clinical evaluation of new drugs to combat parasitic worm infections. Our research spans the entire drug development pipeline — from laboratory discovery to preclinical studies and clinical trials in endemic settings.
We maintain a unique and extensive collection of helminth life cycles to support drug discovery, and we develop assays to screen promising molecules for activity against helminth parasites. Our preclinical research investigates the pharmacokinetics, metabolism and disposition of anthelminthic compounds, while our clinical studies assess the safety and efficacy of candidate drugs and drug combinations in affected populations.
Our team aims to identify and develop novel, broad-spectrum, orally active anthelminthic drugs targeting soil-transmitted helminthiasis, schistosomiasis and food-borne trematodiases — diseases that continue to impose a major global health burden.
Need for Novel Anthelminthics
Soil-transmitted helminthiasis, schistosomiasis and food-borne trematodiases affect hundreds of millions of people, particularly in poor rural communities in low- and middle-income countries. A vaccine is not yet available — and it is unlikely to be in the near future. Consequently, the only way to control morbidity is to administer therapeutic drugs regularly. As very few of these drugs are currently available, developing new anthelminthics that are safe and efficacious is essential.
Our Research Activities
The research conducted in our unit covers a wide range of activities, from drug discovery to development, from bench work to field clinical trials. Our multidisciplinary team comprises technicians, PhD students, postdoctoral fellows and civil servants trained as biologists, pharmacists, epidemiologists and medical doctors.

Jennifer Keiser
Professor, PhD
Head of Unit
+41612848218
jennifer.keiser@swisstph.ch
Highlights

Clinical Development of Emodepside against Soil-Transmitted Helminthiasis
Soil-transmitted helminth infections are caused by different species of parasitic worms, including whipworms, hookworms and roundworms. Worldwide, more than 1.5 billion people are infected with at least one soil-transmitted helminth, with most of the infected population living in sub-Saharan Africa. Safe drugs are available to treat soil-transmitted helminth infections, but the efficacy varies widely. The current treatments recommended by WHO are albendazole and mebendazole. However, in the case of the whipworm Trichuris trichiura, a single dose of these drugs can cure only a handful of infected people. And with drug resistance on the rise, new alternative treatments are urgently needed. Emodepside is an anthelmintic treatment used to date in veterinary medicine. To fill the anthelmintic drug pipeline, Swiss TPH researchers have tested emodepside for the first time in humans infected with soil-transmitted helminths in Phase II trials (see publications in New England Journal of Medicine 2023 and The Lancet 2024). Swiss TPH is working with life sciences company Bayer to develop the drug further, with Phase III trials due to start in 2025. We are also conducting laboratory studies to assess drug combinations with emodepside.

Microbiome Studies
Pharmacomicrobiomic interactions between the human gut microbiota and non-antibiotic drugs have attracted significant research interest due to their proven potential to disrupt treatment outcomes. Despite this, there is a substantial knowledge gap regarding pharmacomicrobiomic interactions of anthelmintic drugs. Our group recently demonstrated a strong association between gut microbiota composition and anthelmintic efficacy against Trichuris trichiura (Nature Communications, 2022). In addition, we described the antibiotic properties of critical anthelmintic drugs against a wide range of bacterial species and their potential to induce cross-resistance to other antibiotics following anthelmintic exposure (Communications Biology, 2024). Our current research is systematically investigating these interactions to understand their contribution to treatment failure and their impact on gut microbial taxonomic and functional diversity. By gaining mechanistic insights into these interactions, we aim to unravel their role in shaping treatment outcomes and improve therapeutic strategies.
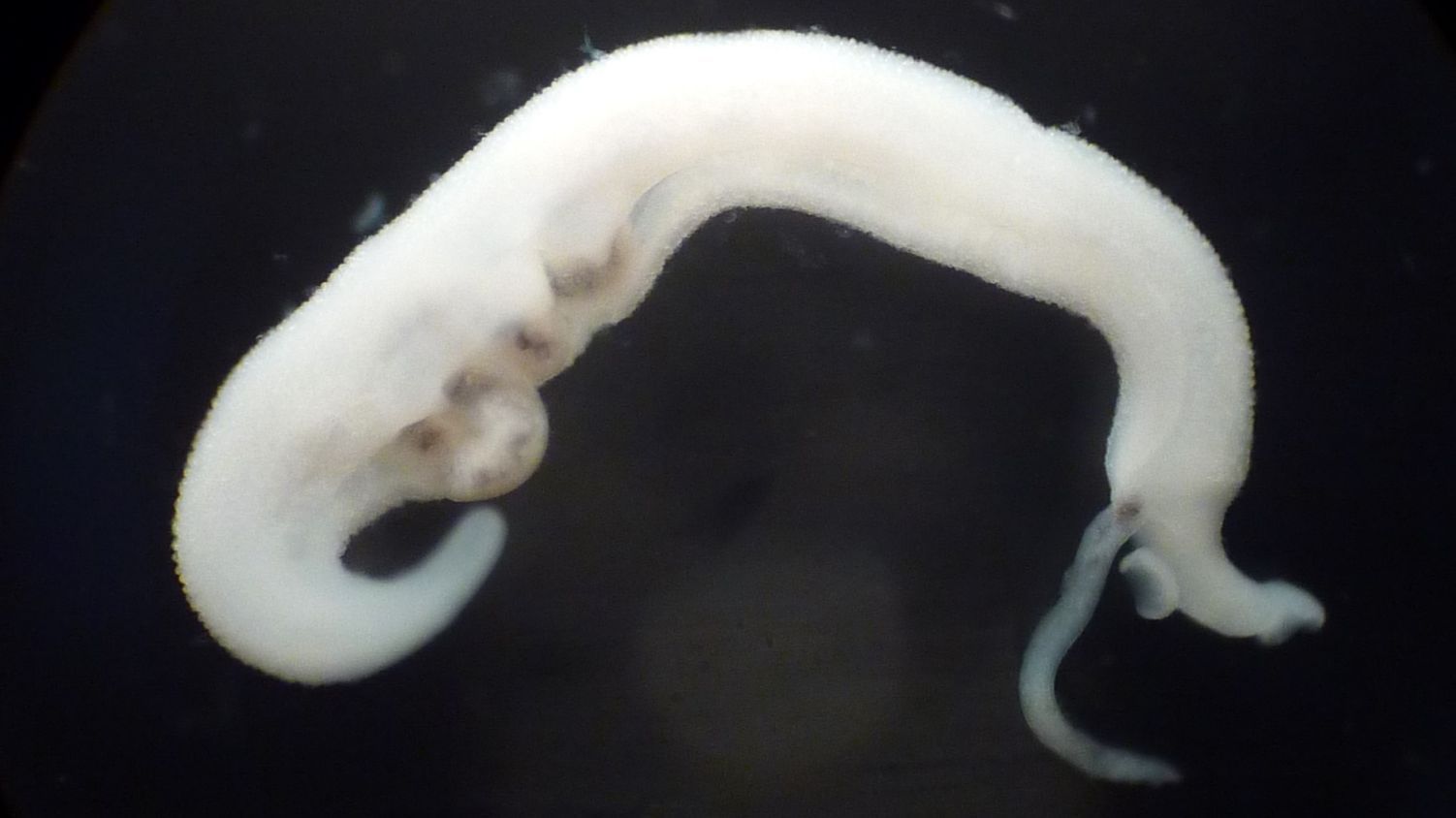
Antimalarials for Schistosomiasis
Schistosomiasis, caused by Schistosoma mansoni, S. haematobium and S. japonicum, continues to affect the health and quality of life of millions of people worldwide. Yet, we still rely on praziquantel as the sole treatment. Drug repurposing (or repositioning) is the development of new indications from existing, failed or abandoned drugs. Particular attention has been paid to antimalarials, as Plasmodium and Schistosoma spp. both utilise hemin as a common food source. The aim of this project is to investigate the antischistosomal properties of the artemisinins in vitro and in vivo, including pharmacokinetic studies. Read more

Novel Tools and Strategies for Breaking Schistosomiasis Transmission
Challenges occur on “the last mile” towards schistosomiasis elimination, including the persistence of transmission hotspots, recrudescence of infection in areas where the prevalence has been successfully reduced, and diagnostics that are not sensitive and specific enough to detect light intensity infections. In the SchistoBreak study, we explore the application of new point-of care diagnostic tools and adaptive intervention strategies that are tailored to the micro-epidemiology of schistosomiasis in the North of Pemba island, Zanzibar, United Republic of Tanzania. Read more
Collaborators
Selected Projects
All ProjectsLatest Publications
All PublicationsAssaré R.K et al. Prevalence, risk factors and trends of human schistosomiasis in Côte d'Ivoire from 1974-2023: systematic review and meta-analysis. Infect Dis Poverty. 2026;15:8. DOI: 10.1186/s40249-025-01410-9
Knopp S et al. Resistance evaluation and surveillance initiative for schistosomiasis treatment: study protocol for the RESIST project. BMC Infect Dis. 2026(in press). DOI: 10.1186/s12879-025-12474-1
Kshirsagar R et al. Bile salt-polymer ternary solid dispersion improves dissolution and in vivo performance: a case study of mebendazole. J Drug Deliv Sci Technol. 2026;115(Part 3):107847. DOI: 10.1016/j.jddst.2025.107847
Lin Y et al. Cost comparison of school-based mass drug administration of albendazole and ivermectin versus albendazole alone for soil-transmitted helminth control in Uganda. PLoS Negl Trop Dis. 2026;20(1):e0013913. DOI: 10.1371/journal.pntd.0013913
Schneeberger P.H.H et al. Profound taxonomic and functional gut microbiota alterations associated with trichuriasis: cross-country and country-specific patterns. NPJ Biofilms Microbiomes. 2026(in press). DOI: 10.1038/s41522-026-00911-1

 Ombeni Ally
Ombeni Ally
 Naomi Chi Ndum
Naomi Chi Ndum
 Maude Dagenais
Maude Dagenais
 Julian Dommann
Julian Dommann
 Jennifer Giovanoli
Jennifer Giovanoli
 Cécile Häberli
Cécile Häberli
 Eveline Hürlimann
Eveline Hürlimann
 Hannah Jeanguenat
Hannah Jeanguenat
 Jennifer Keiser
Jennifer Keiser
 Stefanie Knopp
Stefanie Knopp
 Charlotte Krawczyk
Charlotte Krawczyk
 Gordana Panic
Gordana Panic
 Pierre Schneeberger
Pierre Schneeberger