Group | Viral Immunology
The Viral Immunology group studies human immune responses to viral infection and vaccination using a combination of functional antibody and B cell receptor repertoire analyses. The goal of our research is to characterize immune responses in African compared to U.S. and European populations, with a current focus on responses to SARS-CoV-2 and other human coronaviruses as well as to COVID-19 vaccination. We also develop viral antigen capture assays based on human and mouse monoclonal antibodies that we generate by antigen-specific single B cell sorting and hybridoma technology. Assays will be used to study the epidemiology of viral infections in African populations.
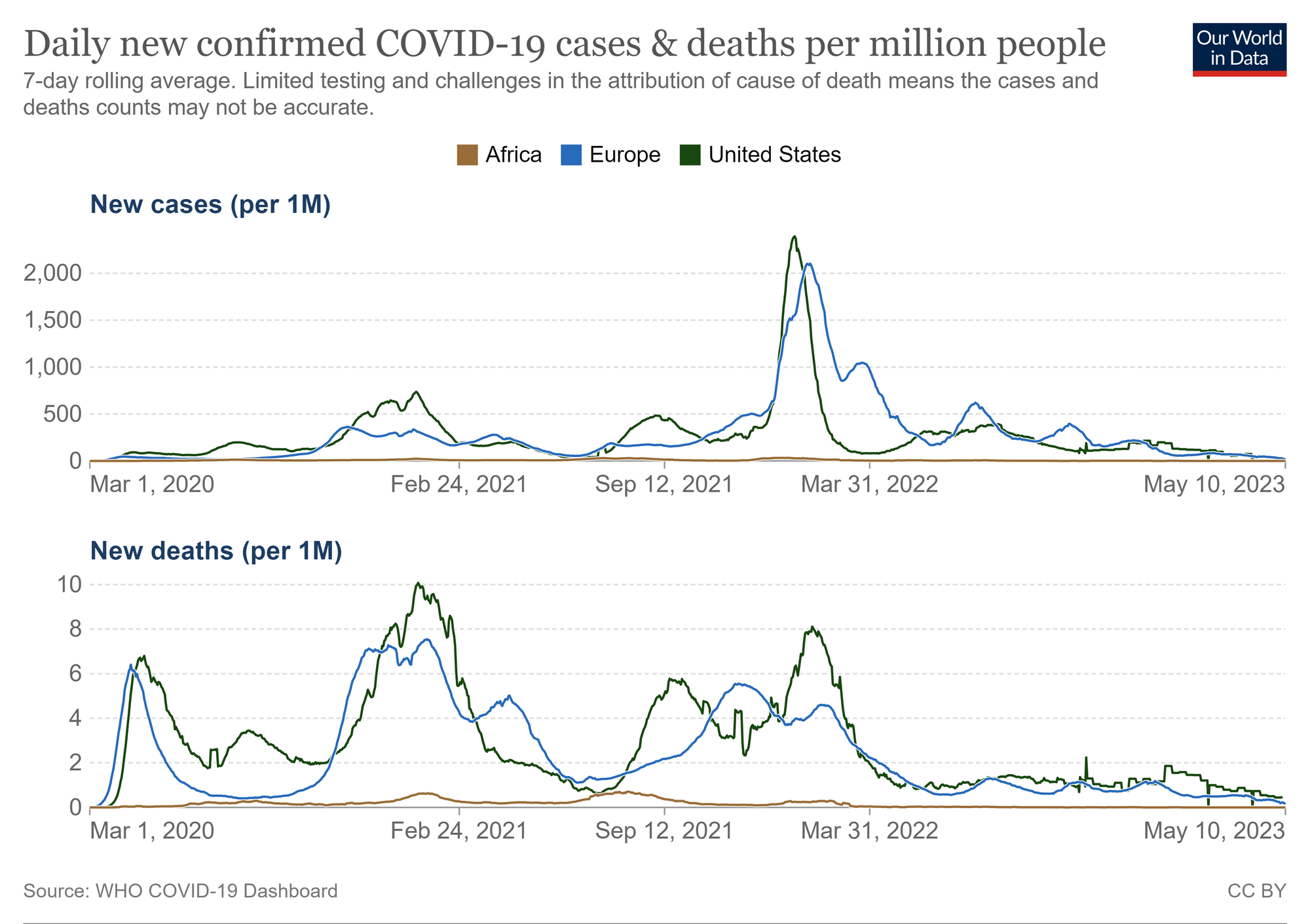
In contrast to soaring COVID-19 case numbers during pandemic waves in the Americas and Europe, large parts of Africa have reported low numbers of severe SARS-CoV-2 infections and COVID-19-related deaths. Hypotheses to explain this observation include differential innate and adaptive immune mechanisms of protection in different populations.
Much has been learnt about immune responses to SARS-CoV-2 in U.S. and European COVID-19 patients, contributing to the development of effective vaccines. Similar studies in African populations with different immunological backgrounds are required to define shared and distinct, potentially beneficial features of immune responses to SARS-CoV-2 and to investigate the nature and longevity of infection- and vaccine-induced immunity to adequately tailor required public health interventions to different populations.

Group Leader
+41612848640
katharina.roeltgen@swisstph.ch
Key Projects
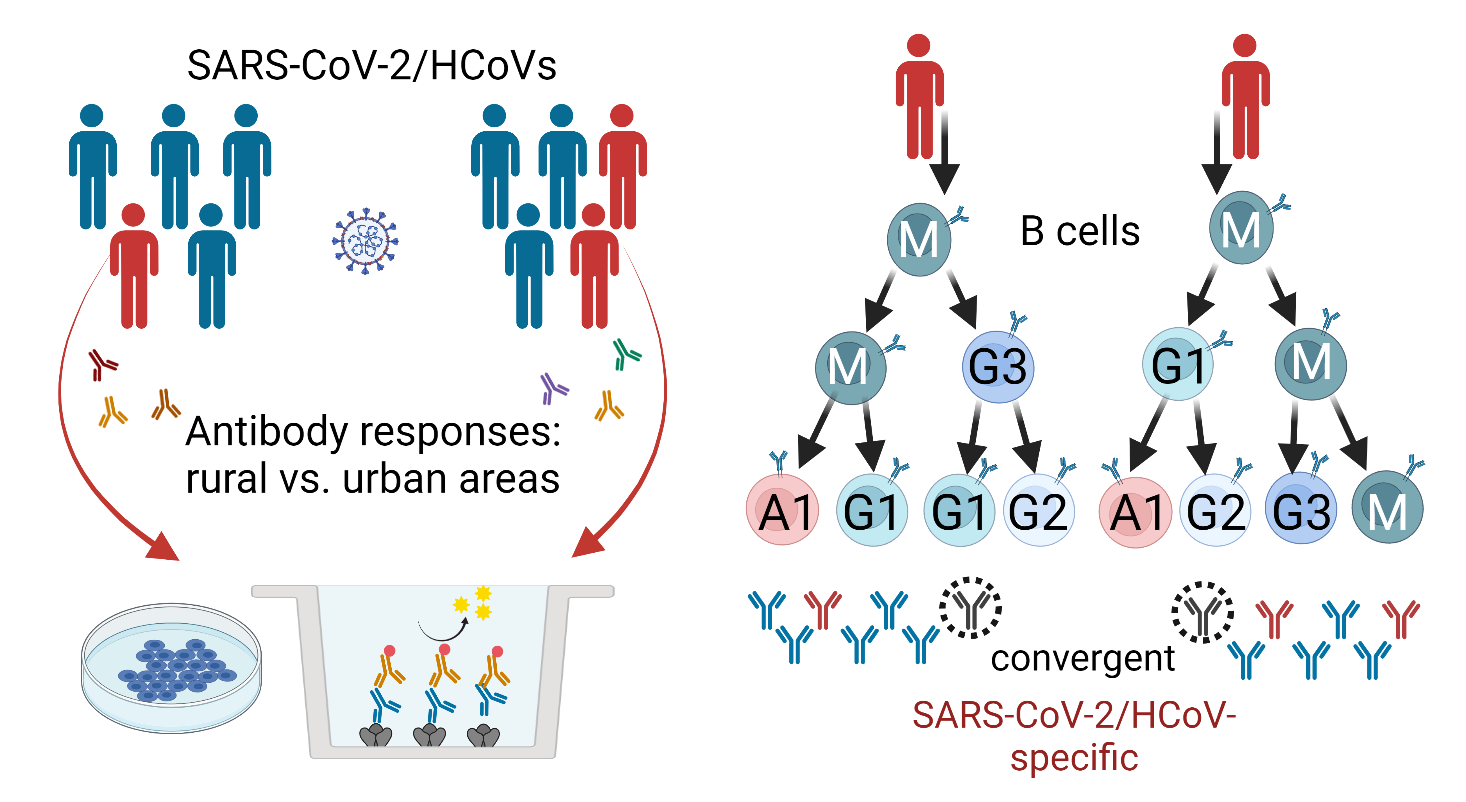
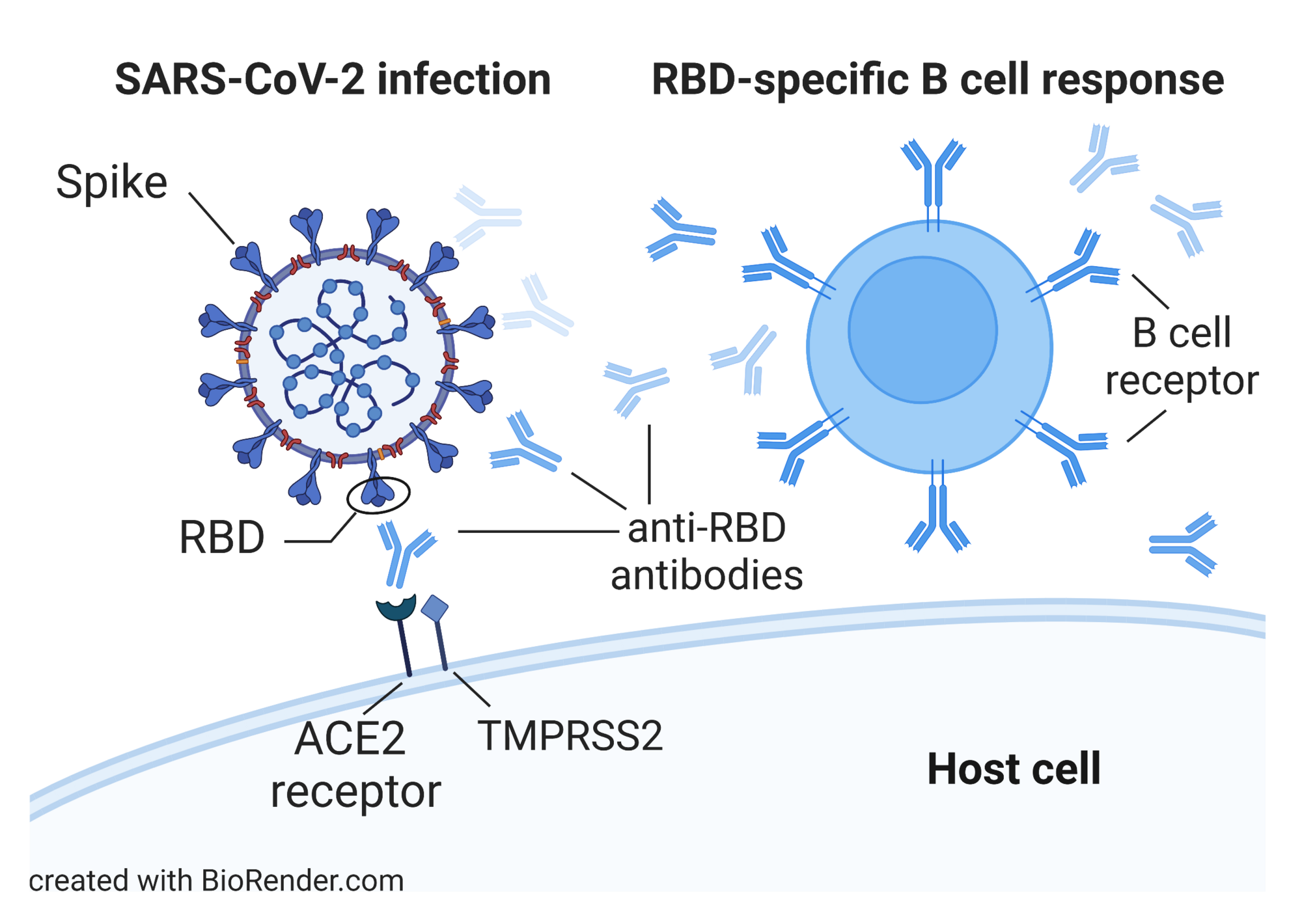
Characterization of B Cell and Functional Antibody Responses to Human Coronaviruses in Africa
The goal of this project is to characterize immune responses of populations in Africa to SARS-CoV-2 and other human coronaviruses (HCoVs) as well as to COVID-19 vaccination. Results will be compared to available data from U.S. COVID-19 patients and vaccinees. Specific objectives are to (i) determine the prevalence and longitudinal development of serum and saliva antibodies to SARS-CoV-2 and HCoVs in individuals from rural and urban areas, (ii) conduct a detailed analysis of antibody specificities and effector functions in samples from study participants, and (iii) analyze clonal B cell lineages in study participants to identify characteristics of class switching, immunoglobulin heavy (IGH) chain gene segment usage, and IGH complementarity-determining region-3 length and somatic hypermutation. Read more
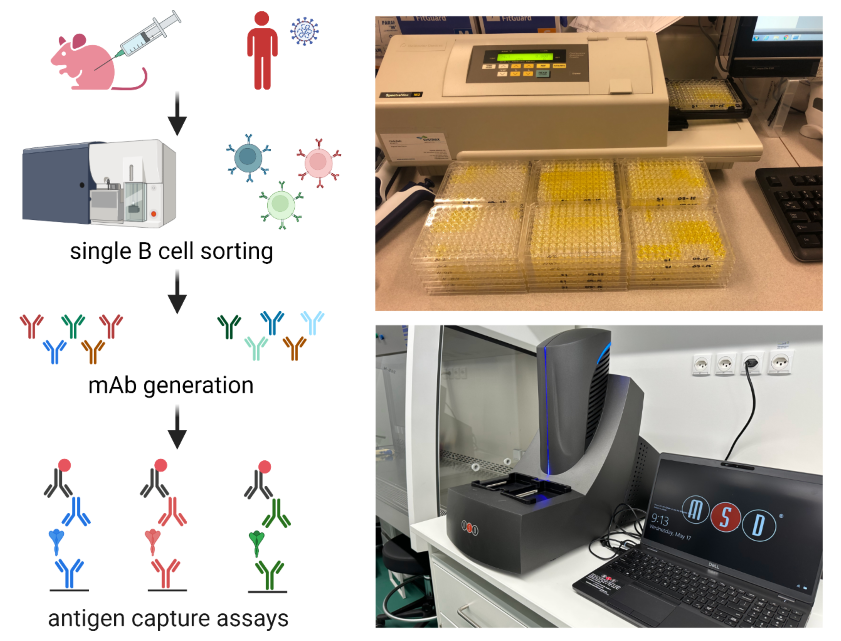
Development of Tools for the Rapid Detection of Past and Present Coronavirus Infection
In the framework of this project we generate human and mouse monoclonal antibodies against different coronaviruses and viral variants using antigen-specific single B cell sorting and hybridoma technology. Monoclonal antibodies will be applied in antigen capture assays for the specific detection of coronavirus and viral variant infection. In addition, we use electrochemiluminescence multi-array technology to detect antibody signatures in plasma to screen populations for previous exposure to different coronavirus variants. Read more
Publications
All PublicationsMontiel-Ruiz M et al. Immune imprinting and antibody profiles to SARS-CoV-2 in urban and rural Ghana. iScience. 2025;28(5):112511. DOI: 10.1016/j.isci.2025.112511
Zaslavsky M.E et al. Disease diagnostics using machine learning of B cell and T cell receptor sequences. Science. 2025;387(6736):eadp2407. DOI: 10.1126/science.adp2407
Hosch S et al. PHARE: a bioinformatics pipeline for compositional profiling of multiclonal Plasmodium falciparum infections from long-read nanopore sequencing data. J Antimicrob Chemother. 2024;79(5):987-996. DOI: 10.1093/jac/dkae060
Jacobson K.B et al. Gestational SARS-CoV-2 infection in a Ugandan birth cohort: high incidence, mild maternal disease, and evidence of association with transient infant stunting. Am J Trop Med Hyg. 2024;111(5):1107-1117. DOI: 10.4269/ajtmh.23-0801
Röltgen K, Boyd S.D. Antibody and B cell responses to SARS-CoV-2 infection and vaccination: the end of the beginning. Annu Rev Pathol. 2024;19:69-97. DOI: 10.1146/annurev-pathmechdis-031521-042754
Gao F et al. Spheromers reveal robust T cell responses to the Pfizer/BioNTech vaccine and attenuated peripheral CD8(+) T cell responses post SARS-CoV-2 infection. Immunity. 2023;56(4):864-878 e4. DOI: 10.1016/j.immuni.2023.03.005
Partners and Collaborators
Prof. Dr. Scott D. Boyd, Boyd Lab at Stanford, US
Prof. Dr. Dorothy Yeboah-Manu, Noguchi Memorial Institute for Medical Research, Ghana