Group | Disease Modelling and Intervention Dynamics
The Disease Modelling and Intervention Dynamics research group develops new mathematical models and methodology applied to diseases with highly complex dynamics. We work to understand how pathogen, host and intervention dynamics combine to prevent disease progression and transmission and to address contemporary issues in infectious diseases and global health. We work in the areas of malaria and respiratory viruses, including supporting understanding and interventions against SARS-CoV-2 during the COVID-19 pandemic.
Using Models to Understand Diseases and How to Intervene
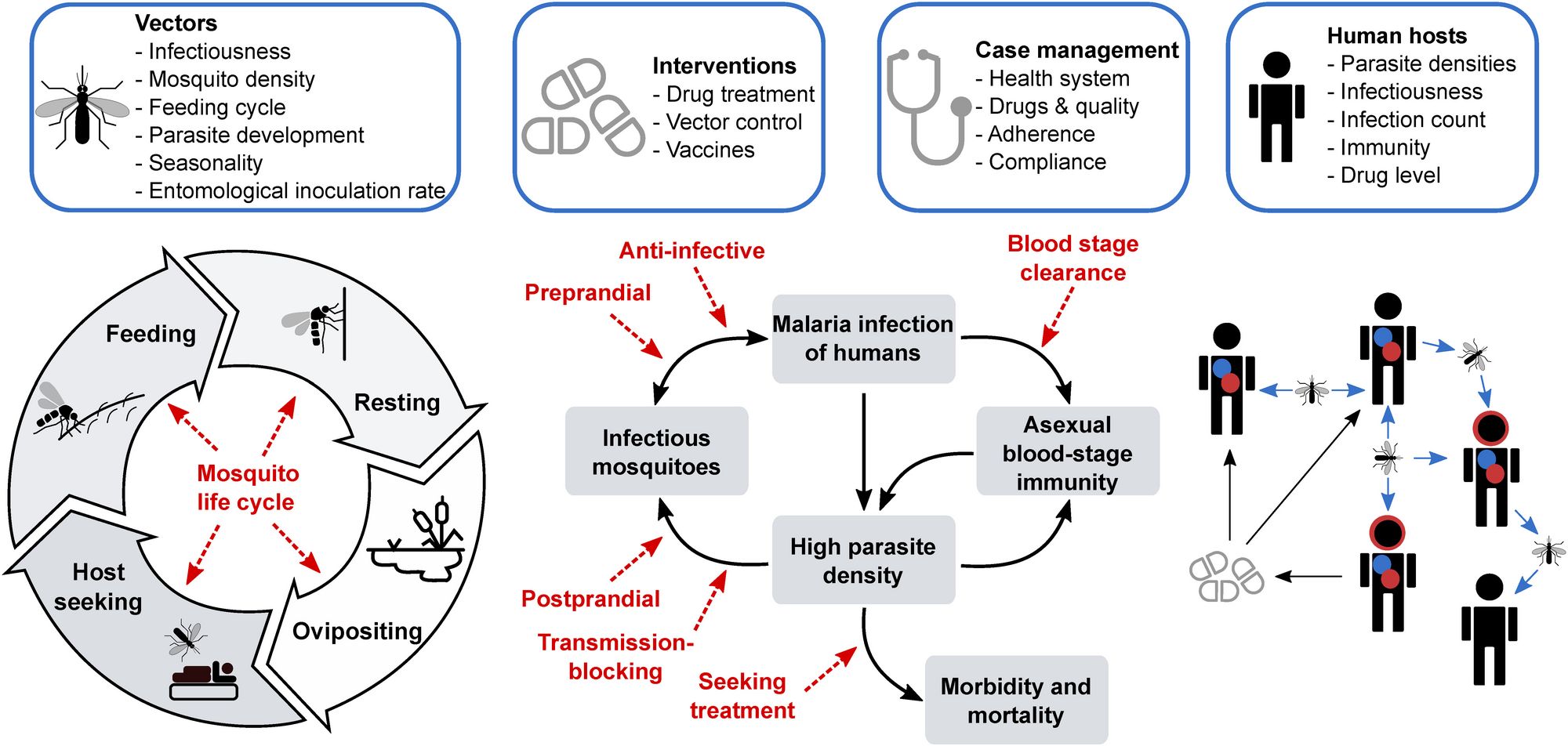
We are an interdisciplinary group of researchers who develop and use models to understand parasitic and viral diseases and inform public health decision-making. We design data-, biology- and epidemiologically-informed mathematical models covering all aspects of disease and treatment dynamics - within-host immune and infection, population transmission, parasite and vector life-cycles, health systems access and interventions as well as detailed interactions with pharmaceutical and non-pharmaceutical interventions.
We use data analysis, and modelling and simulation to examine complex interactions between infectious diseases, individuals and populations, medical interventions and health systems. We apply novel statistical and machine-learning approaches to calibrate and deploy complex disease models.
We use these models to understand disease progression, pathogenesis and disease transmission, and to estimate impacts of existing and novel health interventions at the individuals or population level in the context of real-world health systems. We evaluate potential intervention and resource allocation strategies to achieve effective and equitable impact on infectious diseases, including emerging diseases. We work closely with other research institutions in Switzerland and abroad, as well as with global health donors and public health policy stakeholders.
Generate Evidence for Decision-Making and Development of New Interventions
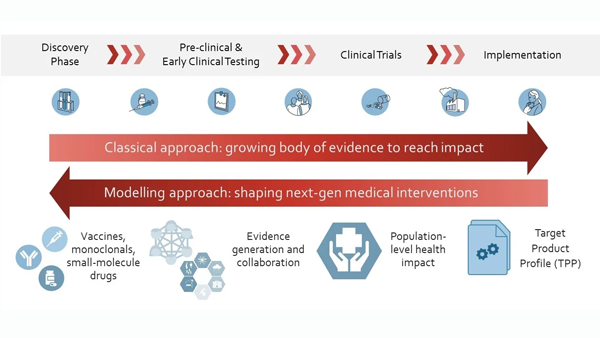
Our main aim is to generate evidence for decision-making along the whole pathway of new intervention development against infectious diseases - from preclinical to clinical testing, and at implementation in real populations and health systems, including for pandemic responses and preparedness. Our model-based evidence supports drug, monoclonal antibodies, vaccine and other intervention development by selecting candidate interventions with optimal properties. Overall, we aim to increase quantitative evidence to support the development and use of impactful interventions, to eliminate, prevent and treat infectious diseases.
Through our close relationships with global health stakeholders, our model-based evidence supports the selection of optimal deployment strategies to achieve disease burden reduction targets, increase prevention or support disease elimination approaches.
Key Projects
Shaping Next-Generation Interventions for Malaria Prevention
Despite progress to reduce malaria burden, malaria parasites are becoming resistant to antimalarial drugs; therefore, new interventions are needed to protect those most vulnerable. However, developing new medical interventions is resource-intensive, and it is often unclear until late in the development process the impact a new intervention will have. Additionally, selection among promising candidates for new antimalarial interventions and immunotherapies must occur early in development. To support this decision-making process, we use mathematical disease models and machine learning tools to define the key performance characteristics for an impactful intervention. We are collaborating with the Bill & Melinda Gates Foundation to bring together R&D, implementation, and global health specialists to define target product profiles for next-generation interventions. Read more
Optimising Disease Elimination Strategies
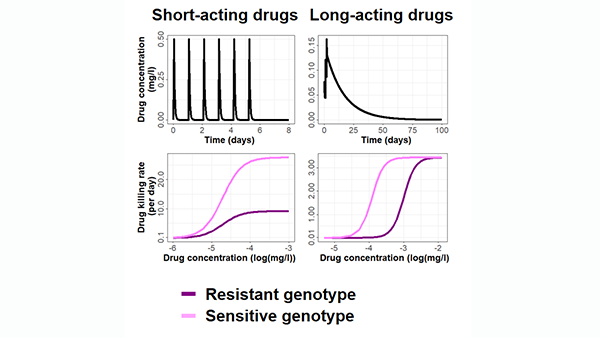
Mathematical models can help us understand disease, and help us plan how to fight disease. This is especially important in the face of diseases that develop resistance to existing drugs and vaccines. Beginning with malaria and COVID-19, our work will provide evidence to select disease control and elimination strategies, and we will extend this to other diseases. We combine data from clinical trials, pre-clinical drug development, information about the disease spreads through the population, about a country’s health system, and how the immune system fights a disease, to predict the optimal way to use drugs and vaccines to fight and eliminate disease. New models in this project also include the evolution of resistant disease strains - this will let us predict the best way to develop and use drugs, vaccines, and other interventions to avoid formation of resistance. Read more
Supporting Strategies for COVID-19 Control
In response to the ongoing COVID-19 pandemic, we developed a new individual-based mathematical model, OpenCOVID, to assess the impact of a range of prevention measures, vaccines, and medical interventions to improve the response and minimise cases, hospitalisations, and deaths in Switzerland and abroad. Our model OpenCOVID has been applied to support Swiss decision-making and predict the public health impact of new emerging SARS-Cov-2 variants of concern. Our model is being used to inform future strategies for ongoing vaccination and response efforts as the world strives to recover from the pandemic's devastating health and economic effects. Read more
Selected Projects
All ProjectsLatest Publications
All PublicationsBraunack-Mayer L et al. Design and selection of drug properties to increase the public health impact of next-generation seasonal malaria chemoprevention: a modelling study. Lancet Glob Health. 2024;12(3):e478-e490. DOI: 10.1016/s2214-109x(23)00550-8
De Salazar P.M et al. Severe outcomes of malaria in children under time-varying exposure. Nat Commun. 2024;15:4069. DOI: 10.1038/s41467-024-48191-7
Le Rütte E.A et al. A case for ongoing structural support to maximise infectious disease modelling efficiency for future public health emergencies: a modelling perspective. Epidemics. 2024;46:100734. DOI: 10.1016/j.epidem.2023.100734
Price D.J et al. Tafenoquine following G6PD screening versus primaquine for the treatment of vivax malaria in Brazil: a cost-effectiveness analysis using a transmission model. PLoS Med. 2024;21(1):e1004255. DOI: 10.1371/journal.pmed.1004255
Prunas O et al. Estimated population-level impact of pneumococcal conjugate vaccines against all-cause pneumonia mortality among unvaccinated age groups in five Latin American countries. J Infect Dis. 2024(in press). DOI: 10.1093/infdis/jiae144

 Lydia Braunack-Mayer
Lydia Braunack-Mayer
 Daniella Figueroa-Downing
Daniella Figueroa-Downing
 Pablo Martinez de Salazar
Pablo Martinez de Salazar
 Thiery Masserey
Thiery Masserey
 Narimane Nekkab
Narimane Nekkab
 Ottavia Prunas
Ottavia Prunas
 Maximilian Richter
Maximilian Richter
 Andrew James Shattock
Andrew James Shattock
 Swapnoleena Ventura Sen
Swapnoleena Ventura Sen