Unit | Clinical Research
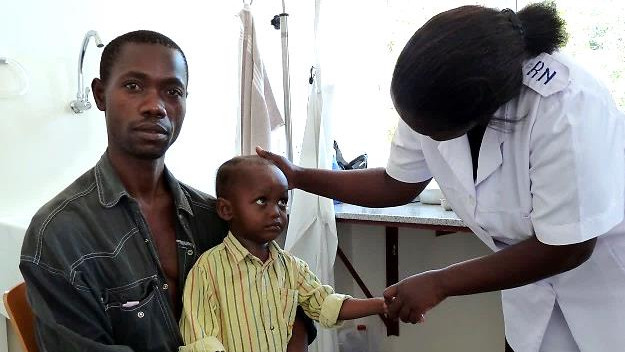
The Clinical Research Unit (CRU) at Swiss TPH designs, conducts, and reports on clinical research studies, primarily in and for low- and middle-income countries, with a focus on vulnerable populations.
In collaboration with international partner institutions, we conduct high-quality scientific studies in the areas of tuberculosis, HIV/AIDS, febrile illnesses, neglected tropical diseases, non-communicable chronic diseases, and mental health. The goal of our work is to gain knowledge about novel clinical tools or interventions, share our findings with the scientific community and the public, inform health policy, and translate knowledge into improved health. We typically work in multidisciplinary and transdisciplinary projects in which building research capacity plays a central role. We provide teaching, training and mentoring for students and young scientists at Swiss TPH and at our partner organizations around the globe.
Main CRU Activities
Related Topics
Related Activities
Latest Publications
All PublicationsBeynon F et al. The Tools for Integrated Management of Childhood Illness (TIMCI) study protocol: a multi-country mixed-method evaluation of pulse oximetry and clinical decision support algorithms. Glob Health Action. 2024;17:2326253. DOI: 10.1080/16549716.2024.2326253
Bresser M et al. Evaluation of COVID-19 antigen rapid diagnostic tests for self-testing in Lesotho and Zambia. PLoS One. 2024;19(2):e0280105. DOI: 10.1371/journal.pone.0280105
Gerber F et al. Same-day versus rapid ART initiation in HIV-positive individuals presenting with symptoms of tuberculosis: protocol for an open-label randomized non-inferiority trial in Lesotho and Malawi. PLoS One. 2024;19(2):e0288944. DOI: 10.1371/journal.pone.0288944
Hiza H et al. Bacterial diversity dominates variable macrophage responses of tuberculosis patients in Tanzania. Sci Rep. 2024;14(1):9287. DOI: 10.1038/s41598-024-60001-0
Keter A.K et al. Simultaneous alleviation of verification and reference standard biases in a community-based tuberculosis screening study using Bayesian latent class analysis. PLoS One. 2024;19(6):e0305126. DOI: 10.1371/journal.pone.0305126

